Table of Contents
Combustion and pyrolysis
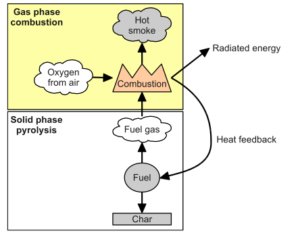
Thermochemical reactions include combustion and pyrolysis. We can say that combustion is an exothermic chemical reaction that produces light and heat as forms of energy and pyrolysis is a decomposition reaction in which organic materials are decomposed when heated. Although both of these processes are thermochemical reactions, there are some differences between them. The primary distinction between combustion and pyrolysis is that combustion occurs in the presence of oxygen, whereas pyrolysis occurs in the absence (or near absence) of oxygen.
Combustion
In general, combustion is a chemical reaction between two or more substances, usually involving oxygen, that produces heat and light in the form of a flame. The rate or speed at which the reactants combine is high, due in part to the nature of the chemical reaction and in part to the fact that more energy is generated than can escape into the surrounding medium, causing the temperature of the reactants to be raised to further accelerate the reaction.
A lit match is a common example of a combustion reaction. Whenever a match is struck, friction heats the head to the point where the chemicals react and generate more heat than can escape into the air, causing the flame to burn. If the heat is blown away by the wind or the chemicals become moist, and friction does not sufficiently raise the temperature, the match will burn out. When a matchstick is ignited properly, the heat from the flame raises the temperature of a nearby layer of the matchstick as well as the temperature of oxygen in the air near to it, and the wood and oxygen react in a combustion reaction. Whenever the total heat energies of the reactants and the total heat energies of the products (including the actual heat and light emitted) reach equilibrium, combustion ceases. Flames have a distinct composition and a complex structure; they are said to be multiform and capable of existing at both extremely low and extremely high temperatures. The presence of excited particles, usually charged atoms and molecules, and electrons, causes light to be emitted in a flame.
Combustion comprises a wide variety of phenomena with widespread application in industry, science, professions, and the home, and the application is based on knowledge of physics, chemistry, and mechanics; their interrelationship is especially evident when dealing with flame propagation.
Pyrolysis
In general, pyrolysis is the chemical decomposition of organic (carbon-based) materials using heat. Pyrolysis, which is also the first step in gasification and combustion, occurs in the absence or near absence of oxygen, and thus differs from combustion (burning), which can occur only when enough oxygen is present. The rate of pyrolysis accelerates as temperature rises. Temperatures in industrial applications are frequently 430 °C (approximately 800 °F) or higher, whereas temperatures in smaller-scale operations may be much lower. Pyrolysis produces two well-known products: biochar (a type of charcoal produced by heating wood) and coke (a product used as an industrial fuel and a heat shield produced by heating coal). Pyrolysis also yields condensable liquids (or tar) and no condensable gases.
Pyrolysis converts organic materials into gaseous components, a solid residue of carbon and ash, and pyrolytic oil (or bio-oil). For removing contaminants from a substance, pyrolysis has two primary methods: destruction and removal. Organic contaminants are broken down into compounds with lower molecular weight during the destruction process, whereas they are not destroyed but are separated from the contaminated material during the removal process. Pyrolysis is an effective method for treating organic materials that “crack” or decompose when exposed to heat; examples include polychlorinated biphenyls (PCBs), dioxins, and polycyclic aromatic hydrocarbons (PAHs). Although pyrolysis cannot be used to remove or destroy inorganic materials such as metals, it can be used to render those materials inert.
Pyrolysis has a wide range of applications in green technology. It can be used to extract materials from goods such as vehicle tyres, to remove organic contaminants from soils and oily sludges, and to produce biofuel from crops and waste products. Pyrolysis can aid in the breakdown of vehicle tyres into useful components, thereby reducing the environmental impact of tyre disposal. Tires are a significant landfill component in many areas, and when burned, they emit PAHs and heavy metals into the air. When tyres are pyrolyzed, they decompose into gas, oil (which can be used as fuel), and carbon black (usable as filler in rubber products, including new tires, and as activated charcoal in filters and fuel cells).
Furthermore, pyrolysis can remove organic contaminants from sewage sludge (semisolid materials that remain after wastewater is treated and the water content is reduced) and render heavy metals remaining in the sludge inert, allowing the sludge to be safely used as fertiliser.
FAQs
What is the process of combustion?
Generally, combustion is a chemical reaction in which a substance reacts quickly with oxygen and produces heat. The original substance is referred to as the fuel, and the source of oxygen is referred to as the oxidizer. The fuel can be solid, liquid, or gas, but it is usually a liquid for aeroplane propulsion.
How is combustion used in industry?
Combustion systems are being used to generate steam and heat for critical manufacturing processes, to heat materials ranging from metals to chemical feedstocks, and to modify the mechanical and chemical properties of materials and products.
For more visit Combustion and Flame – Definition, Types and FAQs






