Table of Contents
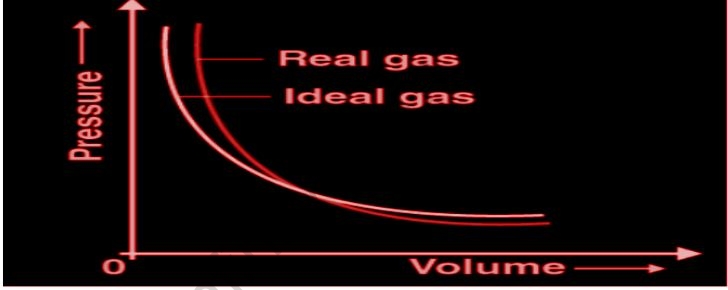
At typical temperatures and pressures, real gasses usually accord with the assumptions of the ideal gas equation to within 5%. Real gasses differ greatly from ideal gas behavior at low temperatures or high pressures. The Dutch physicist Johannes van der Waals explained these aberrations and an equation that may describe the behavior of real gasses across a considerably wider range of pressures in 1873 while looking for a way to relate the behavior of liquids and gasses. Van der Waals noticed that two of the kinetic molecular theory’s assumptions were dubious. Gas particles occupy a minimal fraction of the entire volume of the gas, according to the kinetic theory. It also presupposes that the force is constant.
A brief outline
Deviation from the ideal behavior:
An ideal gas obeys the gas laws under all temperature and pressure conditions. To do so, the gas must follow the kinetic-molecular theory to the letter. The gas particles must have no volume and display no attraction forces toward one another since there is no such thing as an ideal gas because neither of those prerequisites can be met. A genuine gas does not behave as per the kinetic-molecular theory’s assumptions. Real gasses, fortunately, behave very much like ideal gasses when exposed to the temperature and pressure conditions found in a laboratory. Many real gasses behave resembling ideal gasses under normal conditions. For example, near ambient temperature and atmospheric pressure, air, nitrogen, oxygen, carbon dioxide, and noble gases follow the ideal gas law.
Important concepts
The Ideal Gas Law
An ideal gas law follows the ideal gas law:
PV = nRT
P = pressure, V = volume, n = number of moles of the gas, R = gas constant, and T = absolute temperature.
The ideal gas regulation works for every optimal gas, no matter their substance personality. In any case, it is a condition of expression that applies just under specific circumstances. It accepts particles that take part in completely versatile crashes, have no volume, and don’t associate with one another but impact. The gas acts as indicated by the dynamic sub-atomic hypothesis of gasses.
Derivation of real gasses from ideal behavior
An ideal gas consists of small, randomly moving particles that hit elastically. Real gasses are those that do not obey the ideal gas law relations. The notion that as pressure rises, volume decreases causes real gas behavior to deviate from ideal gas behavior. Because the molecules will fill some space that cannot be squeezed any further, the volume will approach zero, but it will not be zero.
The experimental observation of gasses matches the theoretical model perfectly. The problem comes when we determine how well the ideal gas equation, PV = nRT, is used to represent the actual pressure-volume-temperature connection of gasses.
Let’s plot a PV against a V graph to see if this is true. The PV relationship will be constant at a constant temperature. A straight line parallel to the x-axis will be drawn as the PV versus p graph. The graph shown above was created using real data for certain gasses at 273 K.
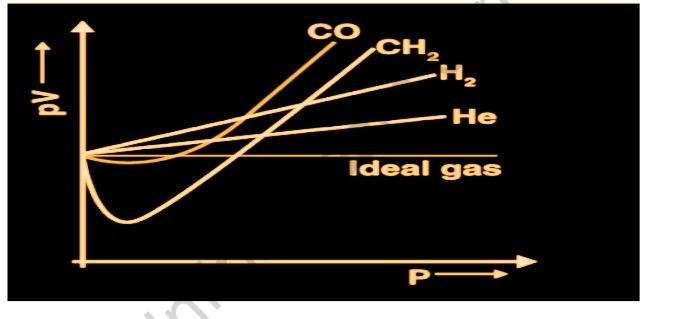
Explain the deviation of real gasses from the ideal behavior
The PV versus p plot for real gasses is not a straight line at a constant temperature, as shown in the graph. There is a major departure from the ideal. When the value of p grows in hydrogen and helium, the value of PV increases.
In some circumstances, such as methane and carbon dioxide, there is a negative departure from ideal behavior at first, but as pressure rises, the value of PV declines and achieves a minimum. After reaching its lowest point, the PV value rises, crosses the ideal gas line, and then continues to exhibit a positive deviation. As a result, at any temperature and pressure, real gasses do not obey the ideal gas equation.
Causes of deviation of real gas from ideal behavior
The volume of the molecules in the gasses and the force of attraction amongst the molecules in the gasses are the two causes that determine how far they deviate from optimum behavior. The collisions between gas molecules are considered to be completely elastomeric.
Significance of real gasses in NEET exam
We at Infinity Learn have made it our main goal to give understudies excellent, pleasant, and enlightening schooling. We trust in a learning style that centers around the hypothetical parts of training as well as on the reasonable employments of things that apply to the NEET test. Accordingly, understudies at boundless learn can deal with information at a much more elevated level during live classes. Understudies could utilize our administrations to enliven their review meetings.
When a gas is compressed under high pressure, the empty space between particles shrinks, forcing the molecules closer together. The presumption that the volume of the particles itself is unimportant becomes less valid as the unoccupied space decreases. The decrease in the kinetic energy of the molecules leads them to slow down when a gas is cooled. The attractive forces between particles are more noticeable when the particles are traveling at slower speeds. Another perspective is that continuing to cool the gas would eventually change it into a liquid, and a liquid is no longer an ideal gas. At low temperatures and high pressures, a real gas deviates from an ideal gas.
Also read: Important Topic of Chemistry: Gas law
Frequently Asked Questions
Which of the gasses deviates the greatest from its ideal state?
We know that ideal gas behavior presupposes that the gasses are small or non-existent in size. Xenon (Xe) is the gas with the largest element size. As a result, it's anticipated that when high pressure and low temperature are combined, xenon is the gas that will deviate the most. The molecules of gasses are near each other under certain of these temperature and pressure levels, and they exert significant intermolecular pressure on one another.
What circumstances can lead to non-ideal gas behavior?
When the temperature is exceedingly low, or the pressure is extremely high, it can lead to non-ideal gas behavior.
Is there such a thing that exists as an ideal gas?
Ideal gasses are those in which the molecules have had no size and collisions are perfectly elastic. The gas molecules have negligible intermolecular forces. An ideal gas is a fictitious concept that does not exist in physical reality.