Table of Contents
Electrochemistry is the part of actual science worried about the connection between electrical potential, as a quantifiable and quantitative peculiarity, and recognizable compound change, with either electrical potential as a aftereffect of a particular substance change, or the opposite way around. These responses include electrons moving between cathodes through an electronically-directing stage (commonly, yet not really, an outside electrical circuit, for example, in electro less plating), isolated by an ionically-leading furthermore, electronically safeguarding electrolyte (or ionic species in a response).
Whenever a substance response is affected by a possible contrast, as in electrolysis, or then again if electrical expected outcomes from a synthetic response as in a battery or power module, it is called an electrochemical response. In contrast to substance responses, in electrochemical responses electrons (and essentially coming about particles), are not moved straightforwardly between atoms, however through the previously mentioned electronically-and ionically-leading circuits, individually. This peculiarity recognizes an electrochemical response from a synthetic reaction.

JEE Foundation Class for 10
JEE Foundation Class for 10 enhances critical thinking and problem-solving skills through engaging activities and advanced learning techniques, ensuring academic excellence.
Electrochemical Cell
An unconstrained compound cycle is the one which can occur all alone and in such an interaction Gibbs free energy of a framework diminishes. In electrochemistry, unconstrained response (redox response) brings about the transformation of substance energy into electrical energy. The converse interaction is likewise conceivable where a non-unconstrained synthetic response happens by providing power. These interconversions are completed in hardware called electrochemical cell.
Sorts of Electrochemical Cell
Electrochemical cell are of two sorts:
- Galvanic cells
- Electrolytic cells
Galvanic Cell
The galvanic cell changes over compound energy into electrical energy i.e, power can be gotten with the assistance of redox response. The oxidation and decrease happen in two separate compartments. Every compartment comprises of an electrolyte arrangement and metallic conveyor which goes about as a terminal. The compartment containing the cathode and the arrangement of the electrolyte is called half cells.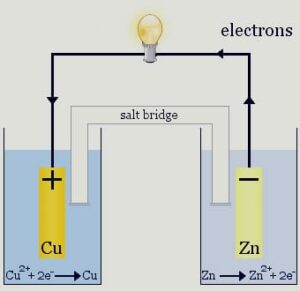
Salt bridge : Salt bridge is generally a reversed U-tube loaded up with a concentrated arrangement of dormant electrolytes. It is utilized to keep up with the charge balance and to finish the circuit by permitting the progression of particles through it. It contains a gel wherein dormant electrolytes like KNO3 or K2SO4 are blended. Through the salt scaffold, negative particle streams towards the anode and positive particle stream to the cathode and the charge balance is kept up with and cell continues to work.
Electrolytic Cell
The electrolytic cell changes electrical energy over to compound energy. Here the terminals are dunked in an electrolytic arrangement containing cations and anions. On providing current the particles move towards terminals of inverse extremity and concurrent decrease and oxidation happen.

Faraday’s Law of Electrolysis
The connection between the amount of electric charge went through an electrolyte and how much substance saved at the terminals was given by Faraday in 1834, as the law of electrolysis.
-
Faraday’s First Law
Whenever an electric flow is gone through an electrolyte, how much substance stored is corresponding to the amount of electric charge went through the electrolyte.
Assuming W is the mass of the substance stored by passing Q coulomb of charge then, at that point, as per this regulation:
W ∝ Q
Presently, Q = I ✕ t
W ∝ I ✕ t
W = z ✕ I ✕ t
Where Z is a steady known as electrochemical same and is normal for a substance stored.
Faraday’s constant(F) – It is the charge moved by 1 mole of electrons and it is equivalent to 96500 coulombs(approx.). As far as Faraday’s steady the quantity of gram likeness electrolyte released at a cathode is equivalent to the faraday’s passed
W = E X Q/96500
-
Faraday’s Second Law
Whenever a similar amount of charge is gone through various electrolytes, then the mass of various substances kept at the separate anodes will be in the proportion of their comparable masses.
Numerically it is addressed as;
W1 /W2 = Z 1/Z 2
Where W1 and W2 are the heaviness of two substances that are stored at their separate anodes and Z 1 and Z 2 are their particular identical weight.

JEE Foundation Class for 9
JEE Foundation Class for 9 enhances critical thinking and problem-solving skills through engaging activities and advanced learning techniques, ensuring academic excellence.
FAQs
What is a half cell or terminal?
It is a metal used to lead electrons in a cell.
Make sense of the capacity of a galvanic cell?
A galvanic cell is an electrochemical cell, used to supply electrical flow by making the exchange of electrons through a redox response. During redox responses, the galvanic cell changes over substance energy into electrical energy by moving energy between the electrons.









