Table of Contents
The cyanide process, as well known as the Macarthur-forest process, has been widely used to extract gold or silver from ores by dissolving them in a dilute solution of potassium or sodium cyanide. Scottish chemists named Robert W. Forrest, John S. MacArthur, and William Forrest pioneered this process in 1887.
One such method consists of three major steps. The finely ground ore is contacted with the cyanide solution in the first step, solids are separated from the clear solution in the second step, and precious metals are recovered from the solution by precipitating with zinc dust in the third step.
History of Cyanide Process
The cyanide process, also known as the MacArthur-Forrest process, is an important method used to extract gold and silver from ores. This process involves dissolving these metals in a solution containing cyanide.
Discovery and Development
- Early Discoveries: In 1783, a chemist named Carl Wilhelm Scheele discovered that gold could dissolve in cyanide solutions. This was the first step toward understanding how to extract gold using cyanide.
- Further Research: In the 19th century, several scientists contributed to this knowledge:
- In 1844, Bagration studied the reactions involving cyanide.
- By 1846, Elsner defined how gold interacts with cyanide.
- Faraday’s research in 1847 further clarified these interactions.
- Invention of the Process: The cyanide process was officially developed in 1887 by Scottish chemists John S. MacArthur and his colleagues Robert and William Forrest. They created a method that allowed for the extraction of gold from low-grade ores by using a cyanide solution.
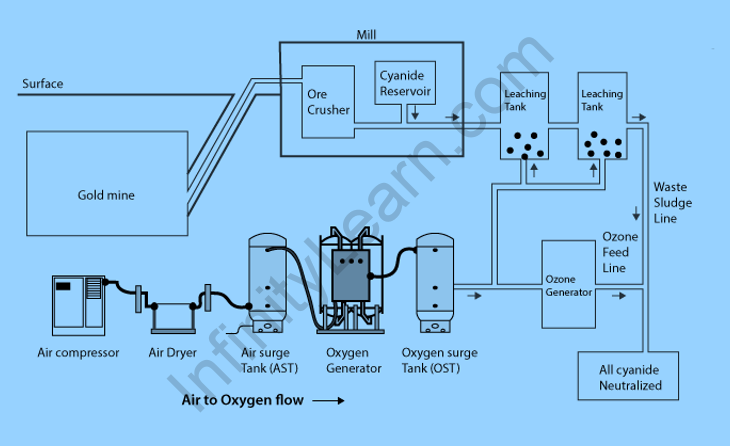
Cyanide Process – Extraction of Gold Through Cyanidation
Gold has been typically found in low concentrations in the form of mined ore. Gold should be separated from the ore’s other minerals. Because gold is insoluble, it must be separated from other minerals before it can be dissolved. As a result, sodium cyanide can be added, where cyanide ions form a complexion with the gold molecules.
- The ore is crushed and ground. If indeed the ore containing the gold contains other metals or sulphide minerals, additional treatments are required before the leaching process can begin. The gold would be mixed with sodium cyanide, which results in Elsener’s equation and reaction shown below. This would be done in order to create soluble gold.
- The gold has become soluble. Leaching is indeed the process of producing soluble gold. A dilute form of sodium cyanide is added to the gold-containing ore during the leaching process. Because gold seems to be soluble after the leaching process, it can pass through the membrane while the rest of the ore cannot. Lime is then added to sodium cyanide to raise the pH to 10-11, thereby favouring the reactants and bringing them to equilibrium.
- The resulting slurry has been treated with activated zinc or carbon to extract gold. Cementation will be the next step, which involves using a zinc electrode with a carbon paste immersed in a solution containing gold cyanide.
Chemical Reactions Involved in the Cyanide Process
The succeeding reactions take place:
At the Cathode –
![]()
Gold is therefore reduced by either gaining electrons or decreasing the oxidation number.
At the Anode –
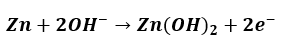
Zinc would then be oxidised in order to lose electrons or increase the oxidation number.
![]()
The cyanide is still present in the tailings. In other words, the slurry produced by the gold leaching process must be recycled or destroyed in some way.
Effects on the Environment from Cyanide Process
Cyanide is a chemical that can have serious negative effects on the environment. It is often used in industries like mining and agriculture, particularly in the processing of crops like cassava. Here are some key points about how cyanide affects the environment:
- Cyanide Contamination: When cyanide is released into the soil, it can harm the soil’s natural balance. This contamination reduces the number of beneficial bacteria and fungi, which are essential for healthy soil. It also alters the soil’s pH and other important chemical properties.
- Microbial Impact: The presence of cyanide can lead to a dominance of harmful microorganisms in the soil, which can cause diseases in plants and animals.
- Water Sources: Cyanide can leach into nearby water bodies through runoff from contaminated soil or direct discharge from industrial processes. This contamination poses a risk to drinking water sources, affecting both human health and aquatic life.
- Aquatic Life: Fish and other aquatic organisms are particularly sensitive to cyanide. High concentrations can lead to mass die-offs, disrupting local ecosystems.
- Human Exposure: People living near cyanide-using industries may be at risk of exposure through contaminated air, water, or food. Symptoms of cyanide poisoning include headaches, dizziness, and in severe cases, death.
- Food Safety: In regions where cassava is processed, improper handling of cyanide can lead to its presence in food products. Consuming cassava with high cyanide levels can be harmful to humans and animals alike.
- Ecosystem Disruption: Continuous exposure to cyanide can lead to long-term changes in ecosystems, affecting plant growth and animal populations. This disruption can reduce biodiversity and alter food chains.
- Degradation of Resources: The degradation of soil and water quality due to cyanide pollution can hinder agricultural productivity, impacting food security and local economies.
FAQs on Cyanide Process
How is gold extracted with cyanide?
Gold is extracted using cyanide in a process called cyanidation. In this method, gold ore is crushed and mixed with a cyanide solution. The cyanide reacts with the gold, dissolving it into the solution. From there, the gold is separated from the solution through further chemical reactions, resulting in purified gold. This method is widely used because it allows for efficient extraction of gold from low-grade ores.
Is cyanide used to clean gold?
Yes, cyanide can also be used to clean gold. In the refining process, cyanide helps remove impurities and other metals from gold, leaving it more pure. However, because of the toxic nature of cyanide, this process is carefully controlled to prevent environmental harm.
Where is cyanide found?
Cyanide is found in both natural and industrial settings. In nature, it exists in certain plants such as cassava, bamboo, and almonds in small quantities. Industrially, cyanide is produced for use in mining, chemical production, and even manufacturing plastics. It is also found in small amounts in cigarette smoke and vehicle exhaust.
How is cyanide extracted?
Cyanide is typically produced industrially through chemical processes. One of the most common methods is the Andrussow process, where methane and ammonia are reacted in the presence of oxygen to produce hydrogen cyanide, which is then purified for various uses.
Who discovered cyanide?
Cyanide was first discovered by Swedish chemist Carl Wilhelm Scheele in 1782. He identified hydrogen cyanide while experimenting with a substance called Prussian blue. His discovery paved the way for understanding cyanide’s properties and its later uses in industrial processes.
Is cyanide still used today?
Yes, cyanide is still used today, particularly in the mining industry for extracting gold and silver. Despite its toxicity, cyanide remains popular because it is an efficient way to separate precious metals from ore. However, its use is regulated, and there are strict safety protocols in place to minimize environmental and health risks.







