Table of Contents
Introduction to Molar Conductivity
Electrochemistry is a crucial topic in the JEE and other competitive engineering exams.
Molar conductivity is a fundamental chemical concept that explains a solution’s ability to conduct electric current.
It is an important parameter for understanding the behaviour of electrolytes in solution, and it is frequently used in a variety of chemical and electrochemical applications.
In this article, we will look at the definition, formula, variation, units, significance, and uses of molar conductivity, offering a thorough knowledge of this important concept in chemistry.
Define molar conductivity
Molar conductivity is the total conducting power of all of the ions formed when a mole of electrolyte is dissolved in a solution. As a consequence, it is never the same.
The total conducting power of all the ions created when a mole of electrolyte dissolves in a solution is defined as molar conductivity. Molar conductivity is a property of an electrolyte solution that is used to determine the electrolyte’s efficiency in conducting electricity inside the solution.
Molar conductivity formula
The molar conductivity formula is mathematically denoted by the expression provided below.
Λm = K / C
K denotes specific conductivity.
C represents the concentration in moles per litre.
In general, the molar conductivity of an electrolytic solution is defined as the conductance of a volume of solution having a single mole of electrolyte that is placed between the two electrodes of unit area cross-section or one centimetre apart.
Unit of molar conductivity
Sm2mol-1 is the SI unit of molar conductivity.
Molar conductance
When electrodes are 1 cm apart and the area of the electrode is large enough that the entire solution is contained between them, molar conductance can be defined as the conductance of all ions created by the dissociation of 1 gram mole of an electrolyte that dissolves in V cc of the solution.
Molar conductance is denoted by μ and can be calculated by multiplying the specific conductance (κ) and volume (V in cc) containing a single mole of the electrolyte as illustrated below:
μ = k x V
where V is the volume in cm3 holding one gramme mole of an electrolyte.
If M is the concentration in gram moles per litre, then a volume containing 1 gram mole of electrolyte has the formula (1000/M). Using V= 1000/M as a value, the preceding equation becomes,
μ = k x 1000 / M
Unit of molar conductance
The unit of molar conductance is Sm2mol-1.
Limiting molar conductivity
The limiting molar conductivity of a solution is it’s molar conductivity at infinite dilution. In another way, when the concentration of electrolytes approaches 0, the molar conductivity is said to be limiting.
Kohlrausch discovered certain trends while studying the limiting molar conductivity of various strong electrolytes. Based on his results, Kohlrausch claimed that the “limiting molar conductivity of an electrolyte can be represented as the sum of the individual contributions of the electrolytes anions and cations.” This is called the Kohlrausch law of independent ion migration.
The limiting molar conductivity of sodium chloride, for example, can be calculated using knowledge of the limiting molar conductivity values of sodium ions and chloride ions.
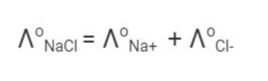
Frequently Asked Questions on Molar Conductivity
What is molar conductivity and its formula?
Molar conductivity is defined as the conductivity of an electrolyte solution divided by the electrolyte's molar concentration, and it evaluates how efficiently a certain electrolyte conducts electricity in solution. The molar conductivity formula is written as: Λm = K / C
How does the molar conductance of strong electrolytes vary with dilution?
The degree of dissociation (the fraction of the total quantity of moles that dissociate into ions) increases with dilution. Molar conductance increases with dilution because the amount of ions increases as dissociation increases.
Can molar conductivity be used to determine the identity of an unknown compound?
Molar conductivity provides information on an electrolyte's strength and behaviour, but it cannot be utilised to determine the identity of a substance that is not known. To identify unknown chemicals, other analytical procedures are usually required.
What is molar conductance and molar conductivity?
Molar conductance is the total conductance of all the ions produced by ionizing 1 g mole of an electrolyte in V mL of solution. The conductivity of one mole of electrolyte is defined as molar conductivity.
What is SI unit of molar conductivity?
Sm2mol-1 is the SI unit of molar conductivity.









