Table of Contents
Introduction to UV
The topic of ultraviolet (UV) light and its diverse applications has generated a lot of interest. In this post, we’ll look at the science behind UV light, its many applications, and how to create equipment that utilizes it.
We’ll take a look at some common UV light methods and applications. So, whether you’re just curious about UV or looking for ways to use it in your business, keep reading!
Principle of UV visible spectroscopy
The Principle of UV Visible spectroscopy: The absorption of ultraviolet or visible light by chemical substances produces different spectra, which is the basis for spectroscopy. The interaction of light and matter is the foundation of spectroscopy.
Transmission, absorption, reflection, and scattering can occur when a substance is exposed to an electromagnetic wave. When the energy difference (E) between a molecule’s ground and excited states equals the energy difference (E), absorption occurs. An electronic transition is the excitation of an electron from its ground state to its excited state.
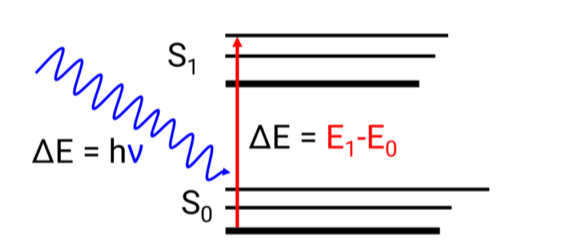
The Planck equation describes the link between energy differential and wavelength.
E=hv=hc/λ
Principle of UV spectroscopy slideshare
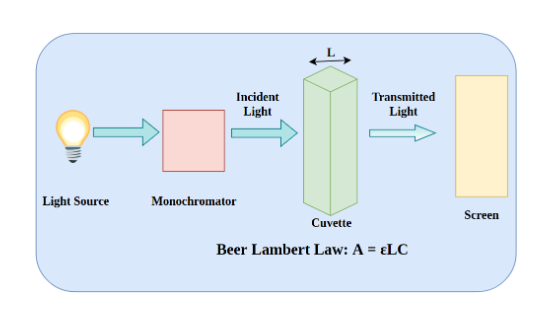
The fundamental property of absorption known as Beer-Lambert’s law underpins UV Visible spectroscopy. The rule governs radiation absorption by an absorbent material.
The Working principle of UV visible spectroscopy slideshare can be used for non-destructive measurements such as detecting the sugar, fat, and protein content of meals and identifying pharmaceuticals by using slides for better presentation.
UV spectroscopy principle beer lambert law
According to the UV spectrometer principle, beer lambert law, a solution’s absorbance is directly proportional to the concentration of the absorbing species within the solution and the route length. UV vis spectroscopy can thus be used to figure out the concentration of an absorber in a solution for a specific route length. This is the basic principle of UV spectroscopy.
Absorbance changes as the concentration increases. This can be derived from references (molar extinction coefficient databases) or, more precisely, from a calibration curve.
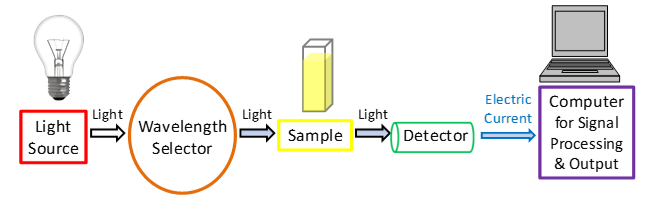
UV spectrophotometer principle (UV principle)
The Principle of UV visible spectrophotometer is based on the fact that Incident light can be absorbed, reflected, or transmitted when it strikes an object. The spectrophotometer measures the amount of light that is absorbed in the UV and Vis ranges. Light transmitted through the sample is measured and compared to an incident light source reference measurement.
The UV spectrophotometer principle can determine the concentration of certain analytes in the sample by applying the Beer Lambert Law, which stipulates that the amount of light absorbed is directly proportional to the concentration of the sample and the path length.
UV principle and calibration
The goal of UV principle and calibration is to ensure the performance, correctness of the results, and dependability of the spectrometer. It is utilized in qualitative and quantitative analyte analysis using Lambert’s law of beer, which is why we frequently need to test instrument performance in-house or by a service engineer.
Also Check Relevant Topics like:
Principle of UV Light
The UV light working principle is explained as follows: When an electrical current is sent through the lamp, the reaction of the elements inside causes UV light to be produced. UV lamps are manufactured to precise requirements based on the materials used and the curing, bonding, or coating required.
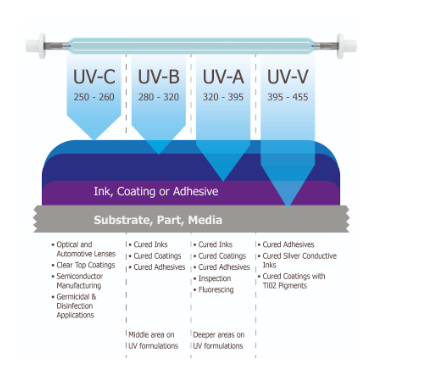
UV lamp working principle
A UV lamp working principle differs from that of a standard lamp in that it is often composed of quartz rather than glass. There is an inert gas mixed with mercury inside. When you plug in the lamp, electricity combines with the mercury, and the lamp emits UV light.
UV transilluminator principle
In molecular biology laboratories, UV transilluminators are used to visualize DNA or RNA that has been separated by electrophoresis across an agarose gel. When the stained gel is exposed to a UV light source, the DNA fluoresces and becomes visible.
UV mutagenesis principle
UV light causes specific changes in the cellular and skin genomes, such as UV-signature and triplet mutations, with the mechanism assumed to require translesion DNA synthesis (TLS) rather than UV-induced DNA base damage.
Wavelength control, absorbance control, stray light limit, and resolving power are all instrument qualification test parameters.
Frequently Asked Questions(FAQs) on Principal of UV
What is the principle of UV spectroscopy?
The principle of UV visible spectroscopy is based on the absorption of ultraviolet or visible light by chemical molecules, resulting in unique spectra.
What is the principle of UV visible spectrophotometer?
The spectrophotometer measures the light intensity of a sample by passing a light beam through it. These tools are used to measure color and monitor color correctness throughout the manufacturing process.
What are the instruments used in UV spectroscopy?
A spectrometer's fundamental components comprise a light source (UV and visible), a monochromator (wavelength selector), a sample stage, and a detector. As a light source, a tungsten filament that is continuous over the UV area is commonly utilized. Detectors are often photodiodes or CCDs.
What is the range of UV spectroscopy?
UV-visible spectrophotometers employ a light source to illuminate a sample with light spanning the electromagnetic spectrum from ultraviolet to visible wavelengths (usually 190 to 900 nm). UV wavelengths typically vary from 100 to 400 nm, while visible wavelengths range from 400 to 800 nm.









