Table of Contents
In the realm of scientific wonders, few elements evoke as much fascination as gold. Its unique properties and versatile applications make it a subject worthy of exploration.
Gold, symbolized as Au in the periodic table, is a dense and shiny yellow metal classified under Group 11 (Ib) and Period 6.
Also Check: Silver
Distinguished as one of the densest metals, gold exhibits exceptional conductivity for both heat and electricity. Remarkably soft, it holds the title of being the most malleable and ductile among all elements. In the measurement convention of troy ounces, a mere ounce of gold can be meticulously hammered into exceedingly thin sheets known as gold leaf, extending up to 187 square feet (approximately 17 square meters).
So, buckle up as we venture into the captivating world of gold, uncovering its scientific properties, revealing intriguing facts, and dissecting the manifold ways it contributes to various scientific fields.

Facts About Gold
Gold’s atomic number 79 on the periodic table defines its distinct characteristics. Its radiant yellow color, unmatched by any other metal, stems from its arrangement of electrons.
Gold (Au), is a precious metal known for its unique chemical composition. Its atomic number 79 signifies the presence of 79 protons in its nucleus. Each gold atom contains 79 electrons orbiting the nucleus, creating a stable structure.
Also check: Mercury Element
This metal is remarkably inert, retaining its integrity and unique properties due to its monoatomic composition. Its pure form, unalloyed with other elements, showcases its distinctive lustrous yellow color and remarkable resistance to corrosion or tarnishing, making it highly coveted in various applications across industries and throughout history.
Unlike most metals, gold does not oxidize or corrode, maintaining its pristine condition over time. This exceptional resistance to corrosion makes gold an exceptional material for scientific instruments and even spacecraft, where durability is paramount.
Furthermore, gold exhibits extraordinary conductivity. Its ability to transmit electricity efficiently without deterioration has made it a crucial component in electronics. From microchips to satellites, the reliability of gold in conducting electricity ensures the seamless functioning of countless scientific devices.
Chemical Properties
Gold, situated in Group 11 and Period 6 of the periodic table, boasts distinctive physical properties.
At a melting point of 1064.18°C (1947.52°F or 1337.33 K), gold transforms from a solid to a liquid state, showcasing its remarkable resilience to high temperatures. As for its boiling point, gold elevates to 2836°C (5137°F or 3109 K) when transitioning from liquid to gas.
Also Check: Facts About Car
Classified in the d-block, gold demonstrates a density of 19.3 g cm⁻³, highlighting its substantial mass in a given volume.
With an atomic number of 79 and a relative atomic mass of 196.97, gold’s identity is defined by its 79 protons and a specific combination of isotopes, notably the key isotope 197Au.
In its solid state at 20°C, gold presents itself as a robust and stable element. The electron configuration of gold, represented as [Xe] 4f¹⁴5d¹⁰6s¹, elucidates the distribution of its electrons in different energy levels.
Collectively, these characteristics contribute to gold’s allure and significance in various scientific and industrial applications.
Golden Insights: Unveiling Scientific Truths
Beyond its physical properties, gold conceals scientific secrets that captivate researchers and enthusiasts alike.
- Atomic Origins: Gold’s origins lie in cataclysmic cosmic events. Elements heavier than iron, including gold, are formed in the fiery hearts of dying stars during supernovae explosions. These astronomical occurrences, billions of years ago, dispersed gold particles across the universe, contributing to its scarcity on Earth.
Also Check: Science About Nitrogen
- Atomic Stability: Gold’s stability at the atomic level is remarkable. Its inertness allows it to remain unchanged even under extreme conditions, making it an ideal material for scientific experiments and studies.
- Nanotechnology Prowess: At the nanoscale, gold particles exhibit extraordinary properties. Gold nanoparticles, due to their unique size-dependent properties, find applications in diverse scientific fields, from biomedical research to environmental sensing.
- Catalytic Abilities: Gold’s catalytic prowess at the nanoscale is a burgeoning area of scientific exploration. Its ability to accelerate chemical reactions holds promise for revolutionizing industrial processes and environmental remediation.
Also Check: Friction
Gold Symbol
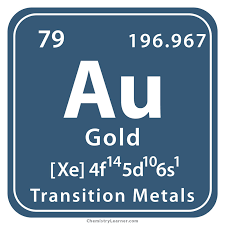
Gold: Compounds
Gold exhibits characteristic oxidation states of +1 (aurous compounds) and +3 (auric compounds). The +1 state is generally unstable, and gold’s chemistry primarily revolves around the more stable +3 state.
Gold is uniquely susceptible to displacement from solution through reduction, surpassing even platinum in its ability to reduce Au3+ ions to metallic gold.
Noteworthy among the limited practical gold compounds are gold(I) chloride (AuCl), gold(III) chloride (AuCl3), and chlorauric acid (HAuCl4). In the first compound, gold assumes the +1 oxidation state, while in the latter two, it adopts the +3 state.
All three compounds play roles in the electrolytic refining of gold. Potassium cyanoaurate (K[Au(CN)2]) serves as the foundation for most gold-plating baths used in the electroplating process.
Additionally, various organic compounds of gold find industrial applications. For instance, gold mercaptides, derived from sulfurized terpenes, are dissolved in specific organic solutions and utilized for embellishing china and glass articles.
Also Check: Speed and Velocity
Uses of Gold
While gold has historically adorned crowns and jewelry, its scientific applications are equally, if not more, captivating.
- Medical Marvels: In medicine, gold nanoparticles show immense promise. Researchers are exploring their use in targeted drug delivery, imaging, and even cancer treatment due to their biocompatibility and unique optical properties.
- Diagnostic Tools: Gold’s exceptional ability to interact with light makes it indispensable in diagnostic tools like lateral flow assays and biosensors. These tools play a pivotal role in medical diagnostics, offering rapid and accurate results.
- Environmental Applications: Scientists leverage gold’s catalytic properties for environmental remediation. Gold nanoparticles serve as catalysts in breaking down pollutants, offering sustainable solutions for cleaning contaminated sites.
- Advanced Technology: The reliability of gold in conducting electricity finds extensive use in advanced technology. From the microelectronics industry to aerospace applications, gold ensures the efficiency and reliability of critical components.
The Scientific Responsibility of Gold Exploration
In the pursuit of scientific advancements involving gold, responsible practices are imperative.
- Sustainable Mining: Responsible gold mining practices are crucial to mitigating environmental impacts. Employing eco-friendly mining methods and reclamation strategies can minimize the ecological footprint of mining operations.
- Ethical Considerations: Ethical sourcing of gold for scientific research involves ensuring fair labor practices and respecting the rights of communities involved in mining activities.
- Waste Management: Proper disposal and recycling of gold-bearing electronic waste are essential to prevent environmental contamination and promote resource conservation.
Also Check: Science Topics for Kids & Students
In the scientific realm, gold’s significance extends far beyond its aesthetic appeal. Its unique properties, from conductivity to stability at the atomic level, make it an indispensable element in various scientific endeavors. Whether unlocking medical breakthroughs or propelling technological advancements, gold continues to shine as a scientific marvel.
By upholding responsible practices in gold exploration and utilization, the scientific community can harness its potential without compromising environmental integrity or social welfare. Embrace the brilliance of gold not just for its allure but for the scientific marvel it represents—an element that has woven its way into the very fabric of scientific exploration and discovery.
Also Check: Stegosaurus
FAQs on Gold
Why is gold called Au?
Gold is called Au because it comes from the Latin word for gold, Aurum.
What is the atomic mass of gold?
The atomic mass of gold is approximately 197 atomic mass units (amu).
Does gold have 79 atoms?
Gold doesn't have 79 atoms on its own; rather, it has 79 protons in its nucleus, giving it an atomic number of 79.
What is the melting point of 22k gold?
22k gold typically melts at around 1,064 degrees Celsius or 1,947 degrees Fahrenheit.
What is the density of 24k gold?
The density of 24k gold is about 19.32 grams per cubic centimeter.









