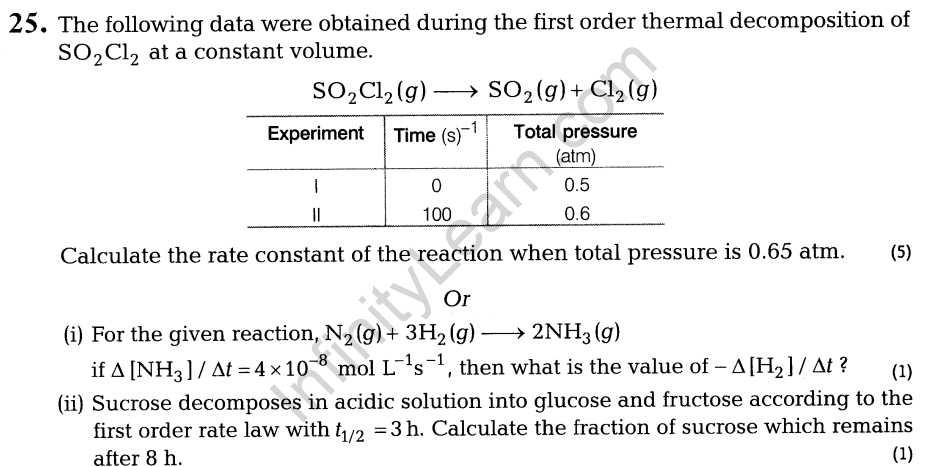
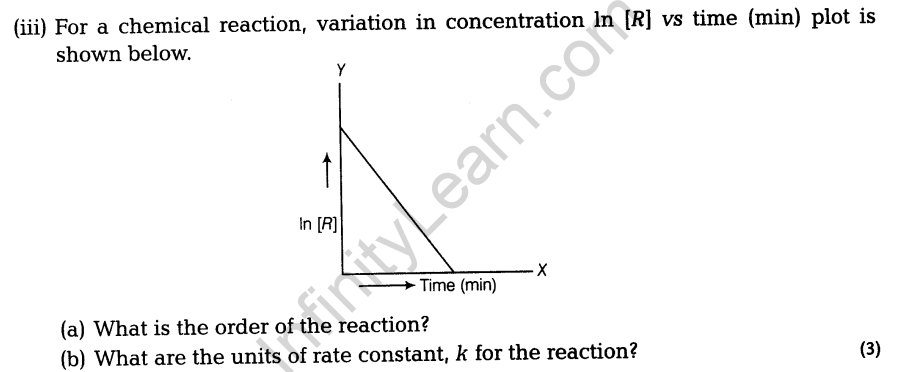
Section A
1.What are the expected products on hydrolysis of lactose?
2.Comment on the nature of two S—O bonds formed in S02 Are the two S—O bonds in this molecule equal?
3.Convert toluene to benzaldehyde and name the reaction.
4.Which of the following is most effective electrolyte in the coagulation of Fe2Os -H20/Fe3+ sol?
KC1, A1C131 MgCl2, K4 [Fe(CN)6 ].
5.A solid AB has CsCl type structure. The edge length of the unit cell is 404 pm. Calculate the distance of closest approach between A+ and B– ions.
Section B
6.Heptane and octane form an ideal solution. At 373 K, the vapour pressure of the two liquids are 105 k Pa and 46 k Pa, respectively. What will be the vapour pressure of the mixture of 25 g of heptane and 35 g of octane?
7.Out of C6H5CHC1C6H5 and C6H5CH2C1, which is more easily hydrolysed by aqueous KOH? Give reason.
8.Why on dilution, the Am of CH3COOH increases drastically, while that of CH3COONa increases gradually?
or
A current strength of 96.5 V is passed for 10 sec through 1L of a solution of 0.1 M aqueous CuS04. Calculate the pH of the solution.
9.Account for the following :
(i) Aniline does not undergo Friedel-Craft’s reaction.
(ii) Gabriel phthalimide synthesis is preferred for synthesising primary amines
10.Give one chemical test to distinguish between the following pairs of compounds.
(i) Ethyl nitrile and nitroethane.
(ii) Benzamide and p-aminobenzoic acid.
Section C
11.(i) Name three anti-depressants.
(ii) Mention one important use of each of the following :
- Sodium benzoate
- Sucralose
12.(i) Write the overall reaction taking place in the process used for the electrolysis of alumina by Hall-Heroult process.
(ii) (a) Extraction of Au by leaching with NaCN involves both oxidation and reduction. Justify, by giving equations for the reactions involved.
(b) What is flux? What is the role of flux in the metallurgy of iron and copper?
13.The treatment of alkyl chloride with aqueous KOH leads to the formation of alcohols, but in the presence of alcoholic KOH, alkenes are the major products. Explain.
14.(i) Write the mechanism of the reaction of HI with methoxymethane.
(ii) What is meant by hydroboration-oxidation reaction? Illustrate it with an example
15.Silver crystallises with face centred cubic unit cell. Each side of this unit cell has a length of 409 pm. What is the radius of silver atom? Assume the atoms just touch each other on the diagonal across the face of the unit cell.
or
Aluminium crystallises in a cubic close packed structure. Radius of the atom in the metal is 125 pm.
(i) What is the length of the side of the unit cell?
(ii) How many unit cells are there in 1cm3 of aluminium?
16. (i) Why cellulose is not digestible?
(ii) Define isoelectric point with an example.
(iii) Write the biological functions of proteins.
17.6 mL of acetic acid (CH3COOH) having density 1.06 g mL 1 is dissolved in 1L of water. The depression in freezing point observed for this strength of acid was 0.0205°C. Calculate the van’t Hoff factor and the dissociation constant of acid. [Kf (H20) = 1.86 Kmol-1 kg]
19. Explain, why
- H20 is a liquid while, in spite of a higher molecular mass, H2S is a gas?
- Iron dissolves in HC1 to form FeCl2 and not FeCl3 ?
- Helium is used in diving equipment?

21.What are polymers? How are polymers classified on the basis of structure?

Section D
23. There is growing interest in the use of chelate therapy in medicinal chemistry. An example is the treatment of problems caused by the presence of metals in toxic proportions in plant /animal systems. Detection of cations through coloured complex formation is done in qualitative analysis.
Read the above passage and answer the following questions.
- What are chelating agents? (l)
- Name the chelating agents that can remove copper, iron and lead from water, (l)
- Name the compound that inhibits the growth of tumours. (1)
- What are the values associated with this chelate? (l)
Section E
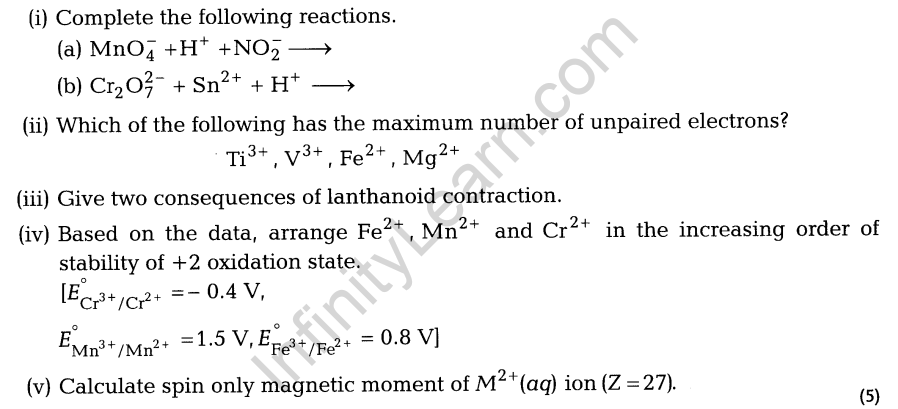
24. Explain the following :
- MnO is basic while Mn2Oy is acidic in nature.
- Chromium is a typical hard metal but mercury is a liquid.
- The transition metals form a large number of complex compounds.
- A substance is found to have a magnetic moment of 3.9 BM. How many unpaired electrons does it contain?
- E° value for the Mn3+/Mn2+ couple is much more positive than that for Cr3+/ Cr2+ or Fe3+/ Fe2+