Table of Contents
Hybridization Of Sulfur Trioxide: SO3 Sulfur trioxide is a compound with the chemical formula SO3. This compound is very important and widely studied as it reacts with water present in the air to produce sulfuric acid.
When this sulfuric acid, in the form of a gas, mixes with rain and falls to Earth, it is called acid rain. Sulfur trioxide is generally colourless and oily but is extremely natural.
Also, it is called battery acid and is used to make acids, fertilizers, lead-acid batteries, metal collections, petroleum refineries, and more. The lewis structure is also called the electron dot structure that determines the number of valence electrons present in an atom.

JEE Foundation Class for 10
JEE Foundation Class for 10 enhances critical thinking and problem-solving skills through engaging activities and advanced learning techniques, ensuring academic excellence.
In addition, they also explain how these valence electrons play a role in bond formation to form molecules. Also, Lewis formation helps to determine the number and nature (single, double, and triple) of the bond shown with the help of lines.
The number of sulfur atoms is 16 which makes its electronic configuration 1s2 2s2 2p6 3s2 3p4.
Since the p shell needs to add 6 electrons, two more electrons are needed to complete the 3p shell. On the other hand, the number of oxygen atoms is eight which makes its electronic configuration 1s2 2s2 2p4.
Also, two more electrons are needed to stabilize the 2p shell. Now, the Lewis structure needs to be designed in such a way that 3p and all 2p shells are completed.
Valence electrons:
The electrons that lie in the outer shell of an atom that is easily involved in bond formation are called valence electrons.
The grip of the nucleus on these electrons is small and the lack of value causes these electrons to participate in bond formation.
Thus, in the case of sulfur trioxide, six valence electrons in each sulfur atom and three oxygen atoms play a role in the formation of one molecule.
Octet law:
According to octet law, the maximum number of electrons that can be filled inside the valence shell is eight.
When it is less than eight electrons, it is only when the atom acquires a bond formation by receiving or donating valence electrons to achieve a stable state such as decent gases.
In both sulfur and oxygen, there is a deficiency of two electrons in each valence.

JEE Foundation Class for 9
JEE Foundation Class for 9 enhances critical thinking and problem-solving skills through engaging activities and advanced learning techniques, ensuring academic excellence.
How can sulfur disobey the law of octet?
The catch is Sulfur which is part 3 of the periodic table.
Sulfur can grow its octet and can hold up to 12 electrons as a result, not following octet law.
Sulfur can do so as it has access to a powerful 3d-subshell to assemble.
Since there is a small power difference between 3p and 3d shells, with the help of a little interesting, an unconnected electron can move from a 3p shell to a 3d shell easily.
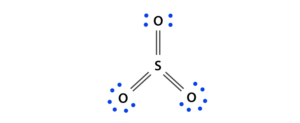
Lewis structure for SO3:
Sulfur trioxide is a chemical molecule of the tetra atom where both sulfur and three oxygen molecules combine with an equal number of valence electrons.
A diagram is drawn showing electron dots of valence around your mark of both sulfur and oxygen atoms with lines that predict bond formation.
The Lewis structure of the sulfur trioxide (SO3) molecule is drawn:
First, look at the total number of valence electrons in one molecule of sulfur trioxide (SO3).
Next, determine how many valence electrons are needed to complete the whole octet in the sulfur trioxide (SO3) molecule.
It is six in one molecule of sulfur trioxide (SO3), where both the sulfur and each oxygen atom require two valence electrons to stabilize their atom.
Next, look at the number and type of bonds that form within a single molecule of sulfur trioxide (SO3). It is a three-dimensional bond between sulfur and each oxygen atom.
Why does SO3 create two bonds?
Due to the distribution of the statutory charge across the atom, double covalent bonds are formed in SO3.
It is determined by the number of electrons the atom delivered – the number of one pair of electrons – is a fraction of the number of electrons in the bond formation.
Therefore, with oxygen, 6-6-1 = 11
And with sulfur, it is 6-0-3 = +3.
Now, the legal charge needs to be stopped in order to achieve a stable status, so having +3 in the middle and -1, in the end, will not happen. It is the reason why three covalent bonds are built on SO3.
Hybridization of Sulfur Trioxide (SO3)
Name: Molecule Sulfur Trioxide
Molecular: Formula SO3
Type of Hybridization: sp2
Bond Angle: 120o
Geometry: Trigonal Planar
Hybridization Of Sulfur Trioxide is sp2. Determined with the help of formulas:
Number of hybrid orbitals = Number of sigma bonds + Number of single pairs
For one double-bonded bond, there is one sigma bond (σ) and one pi (π) bond.
Therefore, the total number of sigma bonds in one SO3 molecule is three, and the total value of one pair of 0 pairs (confirm with Lewis structure).
Therefore, the total number of hybrid orbitals is 3 + 0 = 3. It is in sp2 hybridization that one orbital, as well as two orbital p of the same shell within the atom are scattered and mixed to produce three orbitals new with the same power.
In addition, the Hybridization Of Sulfur Trioxide promotes triangular symmetry with a bond angle of 120 °. In addition, these three new orbital hybrids have 33.33% orbital features and 66.66% p orbital features.
Join Our Online Courses for JEE: JEE Class 12 Online Course | JEE Dropper Online Course
Important Points to Remember:
- In SO3 the sulfur will be the middle atom and will have three oxygen bonds.
- The sigma bond (single bond) is between the sp2 orbital in Sulfur and the other sp2 in Oxygen.
- A double bond is formed between the orbital p orbital of Sulfur and the orbital p orbital of Oxygen.
SO3 is the 3rd period of the periodic table where elements often increase their octet and absorb more than eight valence electrons. It is interesting to note that these behaviors are not different as many factors have these behaviours other than the period of two things. The hybridization in SO3 is sp2 due to the formation of a single sigma bond and a single pi bond.
FAQs
What is SO3 2- hybridization?
In SO3 2-, using the VSEPR model, we can predict tetrahedral electron-pair geometry (and trigonal pyramidal molecular geometry) and from there, predict sp3 hybridization.
Is SO3 sp2?
SO3 hybridization is the atomic sp atom of carbon in the middle. Sp2 of each oxygen atom also.
Why do SO2 and SO3 have the same hybridization?
SO2 hybridization is sp2 of the central sulfur atom. And it is sp2 of each oxygen atom too. Definition of hybridization SO3 including π and sigma bond. SO3 hybridization is the atomic sp atom of carbon in the middle.