Table of Contents
Introduction
The atmospheric chemistry is a branch of atmospheric science that studies the chemistry of the Earth’s atmosphere and the atmospheres of other planets. It is a multidisciplinary research approach that incorporates environmental chemistry, physics, meteorology, computer modelling, oceanography, geology, and volcanology, among other disciplines. Research is becoming more intertwined with other fields of study, such as climatology.
The composition and chemistry of the Earth’s atmosphere are important for a variety of reasons, the most important of which is the interaction between the atmosphere and living organisms. Natural processes such as the volcano emissions, lightning, and bombardment by solar particles from the corona change the composition of the Earth’s atmosphere. Human activity has also altered it, and some of these changes are detrimental to human health, crops, and ecosystems. Acid rain, ozone depletion, photochemical smog, greenhouse gases, and global warming are some of the issues that atmospheric chemistry has addressed. Atmospheric chemists seek to understand the causes of these problems and, by gaining a theoretical understanding of them, to test potential solutions and the effects of changes in government policy.
Also Check: 2024 NEET Rank Predictor
A variety of chemical reactions occur all around us. A flaky brown layer appears on the surface of iron objects such as gates and car bodies. During the Diwali festival, we light crackers that produce bright light and sound. The bright light is produced by the burning of components such as magnesium, which is one of the ingredients in crackers and sparklers. This section will teach us about chemical reactions.
Overview
The atmosphere is made up of a layer of gases that includes nitrogen, oxygen, carbon dioxide, argon, and many other minor gases.
It is made up of 78 per cent nitrogen, 21 per cent oxygen, 0.93 per cent argon, and 0.04 per cent carbon dioxide.
There are four layers in the atmosphere. Let’s take a look at the four different layers of the atmosphere.
1.Troposphere: All biological activities take place in the troposphere. It is the lowest layer, just above the Earth’s surface. Carbon dioxide and water vapours are produced as a result of the reaction in the troposphere. When sunlight passes through the troposphere, the carbon dioxide molecule absorbs a large amount of energy, causing excitation. The troposphere is the lowest region of the atmosphere, just above the earth’s surface, where all biological activity occurs. When sunlight enters this region, the CO2 molecules present absorb a large amount of energy and become excited. This exciting molecule collides with the other molecules, converting the excess energy in them into heat. This heat contributes to global warming.
2. Stratosphere: The formation of ozone is the primary reaction occurring in the stratosphere. Ozone is created in two stages:
Step 1: Oxygen gas dissociation in oxygen atoms.
Step 2: These oxygen atoms combine with additional dioxygen to form ozone.
A chemical reaction is taking place in the mesosphere. As a result of photochemical reactions, free ions and electrons are formed in these reactions. Some of the atom and ion forming reactions that occur with increasing frequency as altitude increases are as follows: Dioxygen ion and electron are formed when oxygen gas decomposes. Nitrogen gas is broken down into dinitrogen ion and electron.
Chemical and photochemical reaction in the atmosphere
Photochemistry is the branch of chemistry that is primarily concerned with the rates and mechanisms of reactions that occur when reactants are exposed to light radiations. The photochemical reaction is, in fact, the thermal reaction of the molecule’s electronically excited state, whereas the dark reaction is the thermal reaction of the molecule’s ground state.
“The photochemical reaction is nothing more than a chemical reaction that begins with the absorption of light as a form of energy.” Temporary peak states would be triggered as the molecules absorb light, and there would be significant physical and chemical property differences from real molecules. With the transfer of hydrogen atoms, electronic charge to separate molecules, protons, and electrons, the resulting chemical structures could be separated, modified, and mixed among similar or different molecules.
When compared to the real ground states, the peak states have stronger reductants and acids. A detailed characterization of the primary events as outlined by the classification of photochemical reaction pathways should ideally be included in the mechanism of a photoreaction.
The quantum yields and thus the rate constants of all relevant photophysical and photochemical processes, as well as information on the structure and fate of any reactive intermediates, as well as their lifetimes and reactivities.
The various chemical and photochemical reactions that occur in the atmosphere are primarily determined by the temperature, composition, humidity, and intensity of sunlight. The absorption of solar radiation in the ultraviolet region causes photochemical reactions in the atmosphere. Photon absorption by chemical species produces electronically excited molecules, which can cause certain reactions. Except at higher temperatures and in the presence of catalysts, these reactions are not thermally possible.
The vast majority of processes observed in nature, on the other hand, are photochemical in nature. Our ability to see things in the world with our eyes is nothing more than a photochemical reaction in which a retinal molecule called rhodopsin (photoreceptor cell molecule) changes shape after being exposed to sunlight or light absorption.
The chemical 7-dehydrocholesterol produced by sunlight is Vitamin D, which is required for bone and tooth development as well as kidney function while aiding skin growth.
The ozone layer in the earth’s stratosphere is formed by the photochemical dissociation of molecular oxygen into oxygen atoms, which then react with oxygen molecules to form ozone.
Photochemical reactions produce ultraviolet (UV) rays that are harmful to human DNA and cause skin cancer.
Photochemical reactions and their peak states have a significant impact on a variety of commercial processes and devices.
Activities that we encounter in our daily lives, such as xerography and photography, are based on photochemical processes, whereas complex activities, such as the manufacturing of semiconductor chips and the printing of newspapers, rely on UV rays.
Chemical reactions involved in the atmosphere
UV light reacts with O2 in the stratosphere, producing nascent oxygen.
Again, ozone absorbs less energetic UV rays, causing cleavage and the formation of O 2 and O. Because of the refrigerants and propellants used, CFCs are also present in the atmosphere. Because CFCs are volatile and do not readily undergo chemical reactions, they remain in the atmosphere for long periods of time.
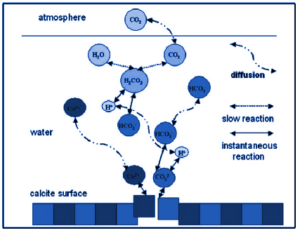
Carbon cycle chemical reactions
The following are the major steps in the carbon cycle process:
- Plants absorb carbon from the atmosphere in order to perform photosynthesis.
- Animals consume these plants, and carbon is bioaccumulated in their bodies.
- When these animals and plants die, carbon is released back into the atmosphere as they decompose.
- Some of the carbon that is not re-emitted into the atmosphere is converted into fossil fuels.
- These fossil fuels are then used for man-made activities, releasing even more carbon into the atmosphere.
Even though carbon dioxide is found in trace amounts in the atmosphere, it plays an important role in energy balance and traps long-wave radiation from the sun. As a result, it acts as a blanket over the planet. If the carbon cycle is disrupted, serious consequences such as climatic changes and global warming will occur. Carbon is a necessary component of all life on Earth. Proteins and lipids, as well as our DNA, are all examples of macromolecules. Furthermore, carbon is the foundation of all known life on Earth. As a result, the carbon cycle, along with the nitrogen cycle and oxygen cycle, is critical to the survival of life on Earth.
FAQ’s
What is an example of a photochemical reaction?
Photosynthesis is the process by which plants use solar energy to convert carbon dioxide and water into glucose and oxygen. Sunlight exposure produces vitamin D in humans. For instance, consider bioluminescence. In fireflies, an enzyme in the abdomen catalyses a light-producing reaction.
What is the fundamental photochemical process?
Photolysis is the process of performing a photochemical reaction. A primary photochemical reaction occurs as a direct result of light absorption. Subsequent chemical shifts are the name given to secondary reactions.
What is the significance of the carbon cycle?
The Carbon Cycle is critical to the survival of all life on Earth. Carbon, from an environmental standpoint, acts as insulation by trapping the heat of the sun. Carbon is the building block of life and forms stable bonds with other elements required for life, according to biology.








