Table of Contents
Physical equilibrium is outlined because the equilibrium develops between completely different phases or physical properties. In these processes, there’s no change in chemical composition. It represents the existence of a similar substance in 2 different physical states. The physical equilibrium will be:
- Phase equilibrium
- Solute-solid equilibrium
- Gas-liquid equilibrium
Equilibrium indicates the constancy of content and composition (as measured by colour, pressure, or temperature) of an item of interest in an exceeding system, no matter the period. Within the Equilibrium state, the speed of the forward reaction is adequate to the rate of the backward reaction.
Types of Physical Equilibrium
Phase Equilibrium:
At 0°C, the quantity of water molecules turning into ice is adequate to the water molecules as the ice melts to form liquid water. The speed of cooling of water is equal to the rate of melting ice. Thus, there’s an equilibrium between solid ice and liquid water.
Ice (s) ⇌ Water (l)
The number of molecules of a liquid changing into vapour is going to be adequate for the number of molecules compressing into liquid during a closed container. The speed of evaporation of liquid water is equal to the rate of condensation of water vapour. The liquid section is in equilibrium with its vapour phase.
Water (l) ⇌ Water (g)
Solute-Solid Equilibrium:
When a solute in an exceedingly|In a very} saturated solution, in reality with an undissolved solute, the number of molecules going (depositing) out of the solution is adequate to the number of molecules getting into (dissolving) from the solid into the liquid. Therefore the solute in a solution is in equilibrium with the undissolved solid.
Solute (aq) ⇌ Solute (s)
Gas-Liquid Equilibrium:
Gases that don’t react with liquid might dissolve directly concerning the pressure within the liquid. In an exceedingly|In a very} closed container, there’s an equilibrium between the gas within the liquid and also the gas present on top of the liquid. In soft drinks, for example, CO2 gas in the liquid is in equilibrium with the gas in the container’s space.
Gas (solution) ⇌ Gas (g)
Chemical Equilibrium
Chemical equilibrium refers to the state of a system within which the concentration of the reactant and, therefore, the concentration of the product don’t change with time, and the system doesn’t show from now on change in properties. Once the speed of the forward reaction is adequate to the rate of the reverse reaction, the state of chemical equilibrium is achieved by the system. Once there’s no further change within the concentrations of the reactants and the products because of the equal rates of the forward and reverse reactions, the system is alleged to be in a state of dynamic equilibrium.
Why is Chemical Equilibrium called Dynamic Equilibrium?
The stage at which the rate of the forward reaction is capable of the rate of backward reaction is named an equilibrium stage. At this point, the number of reactant molecules changing into product and product molecules into reactants is the same. An equivalent equilibrium is distributed with the same reactants anywhere with similar conditions with continuous interchanging of molecules; therefore, chemical equilibrium is dynamic.
Types of Chemical Equilibrium
There are two types of chemical equilibrium:
- Homogeneous Equilibrium
- Heterogeneous Equilibrium
Homogeneous Chemical Equilibrium
In this type, the reactants and the products of chemical equilibrium are all withinside the equal phase. Homogeneous equilibrium may be in addition divided into types: Reactions in which the range of molecules of the products is identical to the range of molecules of the reactants. For example,
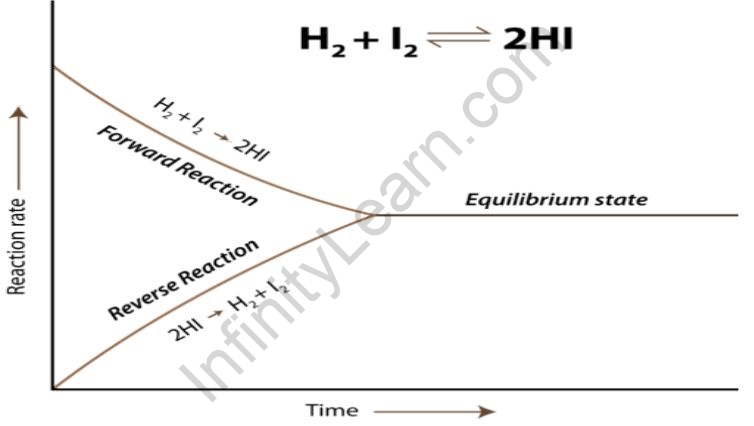
- H2 (g) + I2 (g) ⇌ 2HI (g)
- N2 (g) + O2 (g) ⇌ 2NO (g)
Chemical reactions in which the number of molecules of the products is not equal to the number of molecules of the reactants. For example,
- 2SO2 (g) + O2 (g) ⇌ 2SO3 (g)
- COCl2 (g) ⇌ CO (g) + Cl2 (g)
Heterogeneous Chemical Equilibrium
Chemical equilibrium is characterized by the presence of reactants and products in different phases. Here are a few examples of heterogeneous equilibrium.
- CO2 (g) + C (s) ⇌ 2CO (g)
- CaCO3 (s) ⇌ CaO (s) + CO2 (g)
Hence, chemical equilibrium can be classified according to the phases of reactants and products.
Factors Affecting Chemical Equilibrium
According to Le-Chatelier’s principle, if there may be any alternative withinside the elements affecting the equilibrium conditions, the system will counteract or reduce the impact of the general transformation. This principle applies to each chemical and physical equilibrium.
There are numerous elements like temperature, pressure, and concentration of the system which affect the equilibrium. Some vital elements affecting chemical equilibrium are given below.
- Change in Concentration
- Change in Pressure
- Change in Temperature
- Addition of Catalyst
- Addition of an Inert Gas
Equilibrium in the Physical process is a very reaction, and once there’s a modification in physical state only, it’s referred to as a physical process. Once equilibrium is established in a physical process, it is referred to as physical equilibrium. The characteristics of a system of equilibrium are better understood if we tend to examine some physical processes. The state of equilibrium depends on temperature, pressure, the concentration of reactants and products, and also the presence of different substances. At equilibrium, all the on-top factors are constant. If there is any change in any of the above factors, then it affects the equilibrium also. As a result, the speed of forwarding or backward reaction increases. because the equilibrium is reversible, a brand new equilibrium state is earned within the dynamical direction
Also read: Periodic Trends and Chemical Reactivity
Frequently Asked Questions
Is physical equilibrium possible in a closed system?
It is only possible to achieve equilibrium in a closed system at a particular temperature. The system's measurable properties remain constant when it is at equilibrium.
Why is chemical equilibrium important?
A disruption of equilibrium reactions, such as the binding of oxygen by hemoglobin in carbon monoxide poisoning, can be life-threatening. In contrast, controlling an equilibrium reaction is crucial in chemical syntheses, such as in the production of ammonia.







