Table of Contents
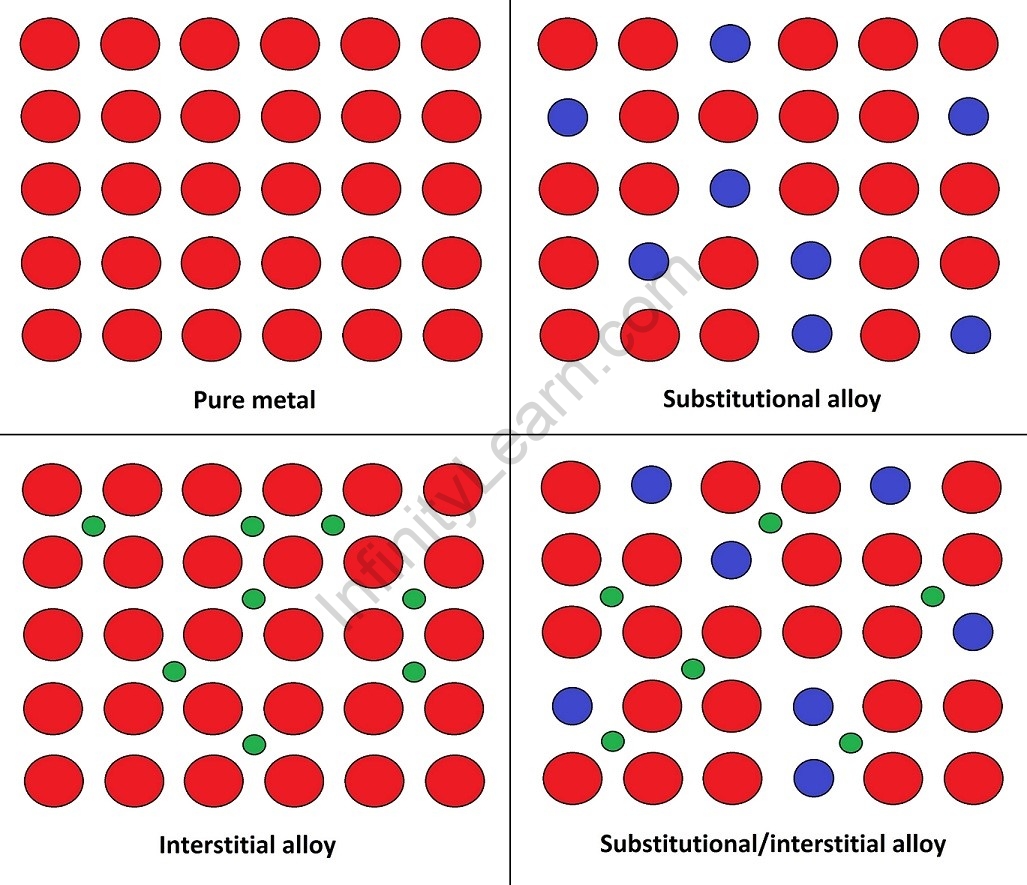
Unlike chemical compounds containing metallic bases, an alloy retains all of the properties of metal in the resulting material, such as electrical conductivity, ductility, opacity, and lustre, but may have properties that differ from those of pure metals, such as increased strength or hardness. In some cases, an alloy can lower the overall cost of the material while retaining important properties. In other cases, the mixture gives the constituent metal elements synergistic properties such as corrosion resistance or mechanical strength. It can be said that alloys are distinguished by their metallic bonding properties. Alloy constituents are typically measured by mass percentage in practical applications, but by an atomic fraction in basic science studies. Alloys are typically classified as substitutional or interstitial alloys based on their atomic arrangement and these can also be homogeneous (consisting of a single-phase), heterogeneous (consisting of two or more phases), or intermetallic. In general, an alloy can be considered as a solid solution of metal elements (a single phase with identical metallic grains (crystals)) or a mixture of metallic phases (two or more solutions, forming a microstructure of different crystals within the metal). Individual pure metals have useful properties such as good electrical conductivity, high strength and hardness, and resistance to heat and corrosion. Commercial metal alloys intend to combine these advantageous properties in order to make metals more useful for specific applications than any of their constituent elements.
Overview
We can say that an alloy is a chemical element mixture that produces an impure substance (admixture) with metal-like properties. An alloy differs from an impure metal in that the added elements in an alloy are well controlled to produce desirable properties, whereas impure metals such as wrought iron are less controlled but are frequently considered useful. Alloys are developed by joining two or more elements, at least one of which must be a metal. The primary metal or base metal is commonly referred to like this, and the name of the metal may also be the name of the alloy. The other constituents may or may not be metals, but they will be soluble and dissolve into the molten base when mixed with it.
Alloys’ mechanical properties are frequently very different from those of their constituents. Aluminium, a metal that is normally very soft (malleable), can be changed by alloying it with another soft metal, such as copper. Despite the fact that both metals are very soft and ductile, the resulting aluminium alloy will be much stronger. A small amount of non-metallic carbon added to iron trades its ductility for the greater strength of an alloy known as steel. Steel is one of the most useful and common alloys in modern use due to its very high strength but still substantial toughness and its ability to be greatly altered by heat treatment. Steel’s corrosion resistance can be increased by adding chromium, resulting in stainless steel, while adding silicon changes its electrical properties, resulting in silicon steel.
Now, the alloying metals involve combining them with one or more other elements. The far more important and common alloying method involves heating the base metal beyond its melting point and then dissolving the solutes into the molten liquid, which may be possible even if the solute’s melting point is much higher than the base’s. Titanium, for example, is a very strong solvent in its liquid state, capable of dissolving most metals and elements.
Alloy formation
An alloy is formed by combining several elements in different proportions. A binary alloy is one that consists of only two components. A ternary alloy has three constituents, whereas a quaternary alloy has four constituents. The properties of the resulting metallic substance differ significantly from those of its constituents. A 1% change in composition can alter the properties of a single unit of an alloy system. The alloy can be classified as follows based on its formation:
- Homogeneous alloy
- The mixture or heterogeneous alloy
Alloy formation of transition (d block) elements
The transition metals are a group of metals found in the centre of the periodic table. The alkaline earth metals, beginning with beryllium, are to the left, and the elements of the boron group are to the right.
These metals have atomic numbers ranging from 21-30, 39-48, 57, 72-80, 89, and 104-112. Many elements, including Zn, Cd, Hg, La, and Ac, have a contentious place in the transition series of elements. La and Ac are also classified as series and actinide series, respectively.
Transition metals have quite a variety of properties and they are harder and less reactive than alkaline-earth metals. They are tougher than post-transition metals. They will create colourful chemical compounds by combining other elements. The majority of them have only one oxidation number. They are electrical conductors in the same way that other metals are.
The atomic sizes of transition metals are indeed very similar, which contributes to their ability to form alloys. Because the atomic sizes are so close, one metal can replace the other in its lattice and form a solid solution. Alloy is the name given to this solid solution. This is why transition metals are miscible with one another in molten form. The corresponding alloy formation occurs as the molten solution cools.
Alloys are prepared in a variety of ways depending on the properties required and the area of application. The following are the most important types and their applications:
Bearing alloy:
Such alloys are created to withstand high pressures when sliding contact is made with another body, such as the rotating shaft of a motor, generator, or vehicle.
Corrosion-resistant:
In this case, noble metals are used. These noble metals initially oxidise and act as a separation layer, preventing any other metals from reacting chemically. Aluminium alloys are the best corrosion resistors.
An alloy is a chemical element mixture in which at least one element is a metal. In fact, the transition metals are harder and less reactive than alkaline-earth metals. The atomic sizes of the d block elements are related to or comparable. Because of this, one metal can easily replace the other and form a new solid, known as an alloy. A homogeneous mixture of two or more metals or metals with nonmetals is referred to as an alloy. As a result, d block elements combine to form alloys.
FAQs
What do you mean by alloy formation?
Alloy is a term used to describe a mixture of metals or a metal with another element. The metallic bond nature of an alloy defines it. It can be either a solid metal solution or a mixture of two or more metallic phases.
What is the process of making alloys?
A large number of alloys are made by combining molten metals, then pouring the mixture into metal or sand moulds and allowing it to solidify. Generally, the main ingredient is melted first, followed by the other ingredients, which should completely dissolve.
Why transition elements can form alloys?
Because the atomic radii of transition elements in the same series are comparable, atoms of one metal in the crystal lattice can easily be replaced by atoms of another metal. As a result, transition metals can easily form alloys under the right conditions.









