Table of Contents
Boyle’s law is an experimental gas law that describes how the pressure of a gas tends to decrease as the volume of the container increases. It is also known as the Boyle–Mariotte law or Mariotte’s law (especially in France). A modern version of Boyle’s law is as follows:
If the temperature and amount of gas remain constant within a closed system, the absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies. Boyle’s law, also known as Mariotte’s law, describes the compression and expansion at a constant temperature. This empirical relationship, proposed by physicist Robert Boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at a constant temperature, i.e., pv = k, a constant. Edme Mariotte, a French physicist, discovered the relationship as well (1676). Under the assumption of a perfect (ideal) gas, the law can be derived from gas kinetic theory (see perfect gas). At sufficiently low pressures, real gases obey Boyle’s law, though the product pv generally decreases slightly at higher pressures, where the gas begins to deviate from ideal behavior.
Gases have a variety of properties that we can observe with our senses, such as gas pressure, temperature, mass, and volume. Scientific observation has revealed that these variables are linked, and the values of these properties determine the state of the gas. Robert Boyle investigated the relationship between the pressure p and the volume V of a confined gas held at a constant temperature in the mid-1600s. The product of pressure and volume, according to Boyle, is approximately constant. For an ideal gas, the product of pressure and volume is exactly a constant.
Overview
Boyle’s law is a gas law that states that the pressure exerted by a gas (of a given mass and constant temperature) is inversely proportional to its volume. In other words, as long as the temperature and quantity of gas remain constant, the pressure and volume of a gas are inversely proportional to each other. Boyle’s law was proposed in 1662 by the Anglo-Irish chemist Robert Boyle. The mathematical relationship between volume and pressure (at constant mass and temperature) for gas is as follows.
P(1/V)
Where P denotes the gas’s pressure and V denotes its volume. By introducing a constant, k, this proportionality can be transformed into an equation.
P=k×(1/V)PV=k
The pressure-versus-volume curve for a fixed amount of gas is kept at a constant temperature below.
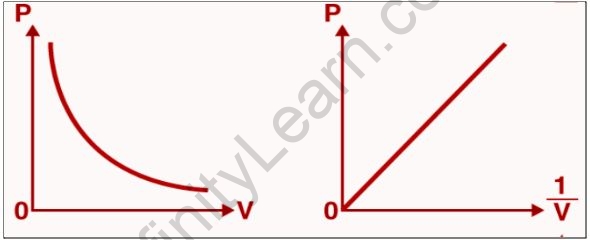
When the pressure exerted by the gas (P) is plotted on the Y-axis, and the inverse of the volume occupied by the gas (1/V) is plotted on the X-axis, a straight line is obtained.
State the Boyle’s law
Boyle’s law describes the relationship between a gas’s pressure and volume at a constant temperature. The volume of a gas is inversely proportional to its pressure under constant temperature.
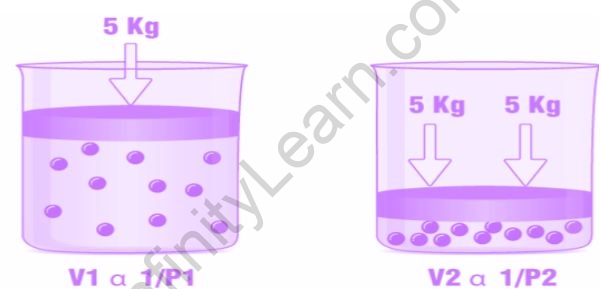
The equation for Boyle’s law is:
V1P
Or
P1/V
PV=k
Where V denotes the volume of the gas, P the pressure of the gas, and K1 the constant, Boyle’s Law, which can also be represented as; can be used to determine the current pressure or volume of a gas.
P1V1=P2V2
Boyle’s law formula
Any change in the volume occupied by a gas (at constant quantity and temperature) results in a change in the pressure exerted by it, according to Boyle’s law. In other words, the product of a gas’s initial pressure and volume is equal to the product of its final pressure and volume (at constant temperature and number of moles). This law can be represented numerically as follows:
P1V1=P2V2
P1 denotes the gas’s initial pressure.
V1 denotes the gas’s initial volume.
P2 denotes the gas’s final pressure.
V2 denotes the gas’s final volume.
The pressure-volume relationship suggested by Boyle’s law can derive this expression. PV = k for a fixed amount of gas kept at a constant temperature. Therefore,
P1V1=k
P2V2=k
P1V1=P2V2
This equation can be used to predict the increase in pressure exerted by a gas on the walls of its container as the volume of the container is reduced (and its quantity and absolute temperature remain unchanged).
Boyle’s temperature
For real gases, the Boyle temperature is described. Real gases do not obey the law of ideal gases, and their particles have volumes. Boyle temperature is defined as the temperature range at which a real gas begins to behave like an ideal gas at a given pressure. A Boyle temperature is when the second coefficient in the expression becomes zero. This Boyle temperature equalizes the attractive and repulsive forces on a gas particle. The Boyle temperature can be calculated using the virial equation of state. The virial coefficients can be used to express Boyle temperature.
Z=1+B/Vm+…
Boyle’s temperature is not the same as Critical Temperature. A gas exhibits non-ideal behavior at the critical temperature. The Boyle temperature is lower than the critical temperature. At Boyle temperature, gas begins to behave like an ideal gas, and the compressibility factor for an ideal gas is one, i.e., Z=1.
Boyle’s law is a fundamental concept in chemistry. This article focused on a detailed examination of what the law is all about and how it works. The article also highlighted key points that you should remember when applying Boyle’s Law. The graphical representation of Boyle’s law is one of the most important things you will see whenever it is discussed. The graphical representation of Boyle’s law is not difficult to grasp; however, we recommend that students reread that section of the article to avoid any confusion.
Also read: Important Topic of Chemistry: Gas law
FAQs
How does Boyle's law operate?
Boyle's law is a gas law stating that the pressure and volume are inversely proportional. When the temperature remains constant, as volume increases, pressure decreases, and vice versa.
What is the significance of Boyle's law?
Boyle's law is important because it describes how gases behave. It demonstrates unequivocally that gas pressure and volume are inversely proportional. When you apply pressure to a gas, the volume decreases while the pressure increases.
What is Boyle's gas law formula?
The empirical relation asserts that at a constant temperature, the pressure (p) of a given quantity of gas changes inversely with its volume (v), i.e., pv = k, a constant, as proposed by physicist Robert Boyle in 1662.
What is a good application of Boyle's Law?
A balloon is an excellent illustration of Boyle's law in action. Blowing air into the balloon causes the pressure of the air to pull on the rubber, causing the balloon to expand. When one end of the balloon is compressed, the pressure rises, causing the balloon's un-squeezed section to expand outward.







