Table of Contents
The critical temperature of a substance is the greatest temperature at which it can be condensed while remaining in a liquid condition. In other terms, the critical temperature is the temperature beyond or below which a substance cannot be liquified out of its vapor or gaseous state, regardless of the amount of pressure applied to it. Tc is the symbol for it. A liquid can only be liquified at a certain temperature, and it becomes more challenging to do so as the temperature rises because the Kinetic Energy of the particles that make up the substance increases as well. As a result, a substance can be transformed from a gaseous state to a liquid state.
A brief outline
The degree of the intermolecular attractions that its particles are exposed to can be determined by a gas’s critical temperature. A gaseous substance with weak intermolecular interactions, for example, will be more difficult to liquefy than one with stronger intermolecular forces of attraction. As a result, the lower the critical temperature, the weaker the intermolecular forces become. Supercritical fluids have progressed from laboratory curiosity to commercially important substances in the last few years. Carbon dioxide, for example, has a low critical temperature (31°C), a low critical pressure (73 atm), and low toxicity, making it simple to hold and regulate. Because numerous chemicals are soluble in supercritical CO2, industrial operations that use it as a solvent are already well established in the oil, food, and other industries.
Important concepts
When we keep raising the temperature of a substance, the molecules begin to move and collide at a rapid rate. At this moment, the density of the substance in its liquefied state decreases, while the density of the substance in its vaporized or gaseous state increases. The vapor pressure rises to the point where the density of the vapor equals the density of the liquid at a specific temperature. As a result, the substance’s vaporized and liquified phases become nearly identical or indistinguishable. This temperature is referred to as the crucial temperature.
The density and numerous other parameters of the liquid, as well as the vapor, become one at this crucial temperature. At this stage, the molecular forces are so strong that no amount of critical pressure will be enough to condense the substance into a calmer, liquid state.
Lower and Upper critical solution temperature
The lower critical solution temperature (LCST), also described as the lower consolute temperature, is the temperature where all components of a composition are miscible. The word lower denotes that the LCST is a lower limit to partial miscibility or miscibility temperature interval for specific compositions only. Polymer solution phase behavior is an important feature in the creation and design of most polymer-related processes. The upper critical solution temperature (UCST) and also the lower critical solution temperature (LCST), both of which rely on the molar mass and pressure, are common solubility boundaries in partially miscible polymer solutions. The system is totally miscible in all proportions at temperatures below LCST, although partial liquid miscibility occurs at LCST.
The critical temperature beyond which the components of a composition are miscible in all proportions is known as the upper critical solution temperature (UCST). The word upper denotes that the UCST is an upper bound to partial miscibility or miscibility temperature range for specific compositions only. Hexane-nitrobenzene combinations, for example, have a UCST of 19 °C (66 °F), indicating that these two compounds are miscible in any quantities above 19 °C (66 °F), but not below.
Water exhibits remarkable behavior when heated outside its critical temperature (647K) and critical pressure (218 atm). The distinction between liquid and gaseous states of water vanishes above the critical temperature, and water has become a supercritical fluid. When water is exposed to temperatures and pressures over the critical point, it loses its capacity to operate as a polar solvent (a dissolving medium). When water is heated to a higher temperature, the molecules are far more likely to get involved with nonpolar molecules.
Significance of critical temperature in NEET exam
For all subjects, Infinity Learn concentrates on materials for younger students. It makes you stride by venture through any point. To start, the master board rapidly covers the essential idea utilizing a few models that permit understudies to interface their hypothetical comprehension to certifiable circumstances. Then, at that point, they gave every one of the essential solutions to questions so understudies could, without much of a stretch, breeze through any test. Mock tests are additionally held by Infinity Learn, and intrigued understudies can take them for nothing. There is likewise an awesome chance to clear any subject or any questions at endless learning. Specialists are close by to help understudies with their interests.
Also read: Heat Capacity and Specific Heat
Frequently Asked Questions
How may we show crucial temperature graphically?
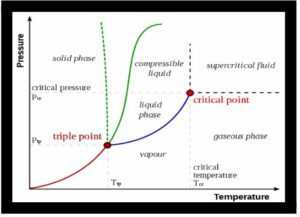
Pressure is represented on the Y-axis, and temperature is drawn on the X-axis in the schematic diagram of critical temperature. This means that the critical temperature can be calculated using the X-axis value. The Y-axis value represents the amount of pressure required to achieve the liquified state of a substance. This occurs when the substance is subjected to a critical temperature. As a result, the obtained pressure is the critical pressure.
What does it mean to have a critical temperature?
The critical temperature of any substance is the temperature above it that can be liquified farther from its gaseous state, regardless of the pressure applied to it.
What exactly do you mean when you say critical point and triple point?
It is said to be a critical point for a substance when its temperature is the critical temperature, and the pressure acting on it is the critical pressure. The triple point occurs when a substance may exist in all three states, i.e., solid, liquid, and vaporized, at a certain temperature and pressure.



