Table of Contents
There is no fundamental difference between cohesion forces and chemical bonds. They are primarily caused by coulombic interactions between charged particles. When the distance between the atoms is less than the sum of the van der Waals radii, repulsion forces become significant. Van der Waals forces are cohesive forces between molecules that are already active over long distances. They are caused by interactions of permanent, induced, or temporary electric dipoles. The latter is referred to as “dispersion forces.” Specific interactions are cohesion forces that are only effective when two molecules’ specific sites come into contact. In fact, specific interactions, such as hydrogen bonds, are short-range site-bounded cohesion forces that significantly weaken one of the partners’ chemical bonds. The distance between the proton and the nearest nucleus of B in the A-H. B hydrogen bond is much shorter than the sum of the van der Waals radii, the distance A-H is greater than in the unperturbed molecule, and the cohesion energy is intermediate between that of pure dispersion forces in the liquid state and that of normal chemical bonds. Hydrogen bonds appear as an intermediate step in the proton transfer from AH to B. A new chemical bond, BH+, is formed during this transfer. H-bonds already share two chemical bond properties: stoichiometry and directionality. Their lifespan, on the other hand, is extremely short. These properties are also shared by so-called n-EDA bonds. The difference in proton affinity of B and the anion A governs the energy of a hydrogen bond—a quantitative expression is proposed. The study of intermolecular forces begins with macroscopic observations that indicate the presence and action of forces at the molecular level. Non-ideal-gas thermodynamic behaviour, as reflected by virial coefficients, vapour pressure, viscosity, superficial tension, and absorption data, is among these observations.
Overview
Intermolecular forces are forces that exist between molecules. The strength of attractive forces between molecules is related to the physical properties of melting point, boiling point, vapour pressure, evaporation, viscosity, surface tension, and solubility. Intermolecular Forces are the name given to these attractive forces.
Intermolecular forces (IMF) are the attractive and repulsive forces that exist between the molecules of a substance. These forces act as a bridge between the interactions of individual molecules in a substance. The majority of matter’s physical and chemical properties are determined by intermolecular forces. Forces exist between molecules as well, and these are referred to collectively as intermolecular forces. The physical properties of a substance are primarily determined by intermolecular forces. The condensed states of matter are caused by intermolecular forces. Intermolecular forces hold the particles that makeup solids and liquids together, and these forces affect a number of the physical properties of mater in these two states.
Molecules cohere despite the fact that their ability to form chemical bonds has been exhausted. The fact that gases can be liquefied, that ordinary liquids exist and require a significant amount of energy to vaporise to a gas of independent molecules, and that many molecular compounds exist as solids all provide evidence for the existence of these weak intermolecular forces. The role of weak intermolecular forces in gas properties was first investigated theoretically by the Dutch scientist Johannes van der Waals, and the term van der Waals forces is used interchangeably with intermolecular forces. Weakly bonded clusters of molecules (such as an argon atom in association with a hydrogen chloride molecule) can exist under certain conditions; such delicately bonded species are known as van der Waals molecules.
Intermolecular interactions
Intermolecular interactions occur when two or more molecules interact, whereas intramolecular interactions occur when atoms within a molecule interact. Intermolecular interactions occur in all states of matter between all types of molecules or ions. They range from strong long-distance electrical attractions and repulsions between ions to relatively weak dispersion forces that have yet to be fully explained. The various types of interactions are classified as follows (in decreasing order of strength): Except at extremely low temperatures, the condensed forms of matter (liquids and solids) would not exist without these interactions. We’ll look at these different forces and interactions in the gas phase to figure out why some materials vaporise at very low temperatures while others remain solids or liquids at extremely high temperatures.
Ion – Ion Interactions
The interactions between ions (ion-ion interactions) are the most straightforward: like charges repel each other, while opposite charges attract. In the gas phase, Coulombic forces operate over relatively long distances. The force is proportional to the product of the charges Z1, Z2divided by the square of the separation distance d2
F=-Z1Z2/d2
Two oppositely charged particles flying around in a vacuum will be attracted to each other, and the force grows stronger as they approach until they eventually stick together and a significant amount of energy is required to separate them. They combine to form an ion-pair, a new particle with a positively and negatively charged area. Because of the relatively strong interactions between these ion pairs and free ions, the clusters tend to grow and eventually exit the gas phase as a liquid or solid (depending on the temperature).
Intermolecular forces of attraction
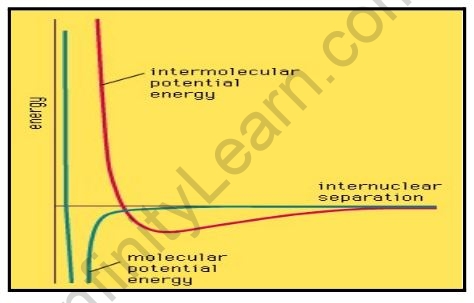
There are many different types of intermolecular forces; the repulsive force and four different types of attractive force are covered here. The energy of interaction varies with distance in general, as illustrated by the graph in Figure. Attractive forces dominate until the two molecules come into contact, at which point strong repulsive forces kick in and the potential energy of the two molecules skyrockets. The shape of the intermolecular potential energy curve depicted in the illustration is similar to that of the molecular potential energy curve depicted in Figure. The minimum of the former, on the other hand, is much shallower, indicating that forces between molecules are typically much weaker than the forces responsible for chemical bonds within molecules.
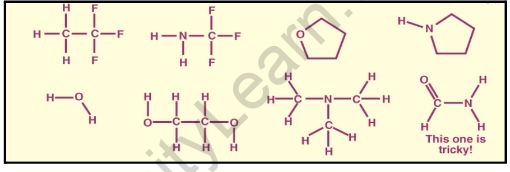
Intramolecular hydrogen bonding examples
Intramolecular hydrogen bonding refers to hydrogen bonding that occurs within a molecule. It occurs in compounds with two groups, one containing a hydrogen atom linked to an electronegative atom and the other containing a highly electronegative atom linked to a less electronegative atom of the other group. The bond is formed by the hydrogen atoms of one group bonding with the more electronegative atom of the other group.
Hydrogen bonds that occur within a single molecule are referred to as intramolecular hydrogen bonds. This happens within a molecule between two functional groups.
For example, ortho-nitrophenol and salicylaldehyde.
Problem: Determine which of the following molecules could form Hydrogen Bonds with other molecules of the same type.
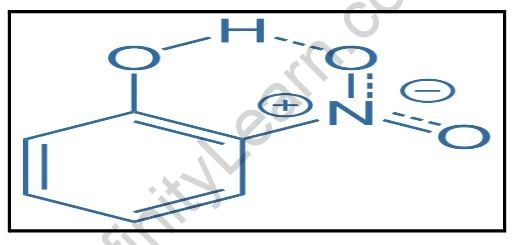
The O atom in the –OH group in the O-nitro phenol molecule is more electronegative than the H and thus -. In contrast, the H atom is +. As a result, the O atom in the –OH group serves as the H donor, whereas the O atom in the nitro group serves as the H acceptor.
Types of intermolecular forces
An intermolecular force is an attractive force that exists between the positive (or protons) components of one molecule and the negative (or electrons) components of another molecule. This force influences the physical and chemical properties of a substance. The boiling point of a substance is proportional to the strength of its intermolecular forces; the higher the boiling point, the stronger the intermolecular forces. We can compare the strengths of intermolecular forces by comparing the boiling points of different substances. This is due to the fact that the heat absorbed by the substance at its boiling point is used to break these intermolecular forces and convert the liquid to vapour.
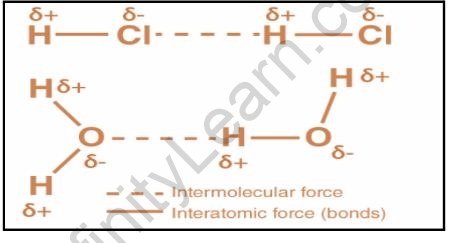
Intermolecular forces are determined by the following interactions:
Interactions between dipoles
Dipole-dipole interactions are attractive forces that exist between polar molecules. Polar molecules have permanent dipoles that form as a result of differences in the electronegativities of the atoms connected by a covalent bond. The partially positive portion of one molecule attracts the partially negative portion of another. In HCl molecules, for example, dipole-dipole interactions occur. Because chlorine is more electronegative than hydrogen, it acquires a partial negative charge (whereas hydrogen acquires a partial positive charge). The dipole-dipole interaction between the HCl molecules then occurs.
Interactions between Ions and Dipoles
Except that they occur between ions and polar molecules, these interactions are similar to dipole-dipole interactions. When NaCl is mixed with water in a beaker, the polar H2O molecules in the beaker are attracted to the sodium and chloride ions. The strength of this interaction is determined by:
- The dipole moment’s magnitude
- The polar molecule’s size
- An ion’s size and charge
Dipole Interactions Caused by Ions
A non-polar molecule is polarised by an ion placed nearby in this type of interaction. When non-polar molecules acquire a charge, they behave as induced dipoles. Ion-induced dipole interaction refers to the interaction between an ion and an induced dipole.
Interaction of Dipoles Induced by Dipoles
These interactions are similar to those observed in ion-induced dipole interactions The difference is that non-polar molecules are transformed into induced dipoles in the presence of a polar molecule nearby.
London Forces or Dispersion Forces
It has the shortest range and is the weakest force. This type of force is caused by electron movement, which creates temporary positive and negative charged regions.
FAQs
What exactly are the four intermolecular forces?
The four basic intermolecular forces are ion-ion interactions, dipole-dipole interactions, hydrogen bonding, and London dispersion forces.
What are intermolecular forces and what are some examples?
Intermolecular forces are the forces that act between molecules. Intramolecular forces, on the other hand, act within molecules. The strength of intermolecular forces is less than that of intramolecular forces. The London dispersion force, dipole-dipole interaction, ion-dipole interaction, and van der Waals forces are all examples of intermolecular forces.










