Table of Contents
When an atom, molecule, or ion loses one or more electrons in a chemical reaction, this is oxidation. When a chemical species oxidizes, its oxidation state increases. Oxidation does not always require the presence of oxygen!
Originally, the term was applied when oxygen resulted in electron loss in a reaction. The modern definition is broader. The loss of electrons by a molecule, atom, or ion during a reaction is referred to as oxidation. When the oxidation state of a molecule, atom, or ion increases, this is referred to as oxidation. The inverse process is called reduction, and it occurs when electrons are gained, or the oxidation state of an atom, molecule, or ion decreases. As an older method of oxidation, oxygen has been added to a compound. Although oxygen is typically added to a compound to meet the requirement for electron loss and an increase in oxidation state, the oxidation concept has been expanded to include additional chemical reactions.
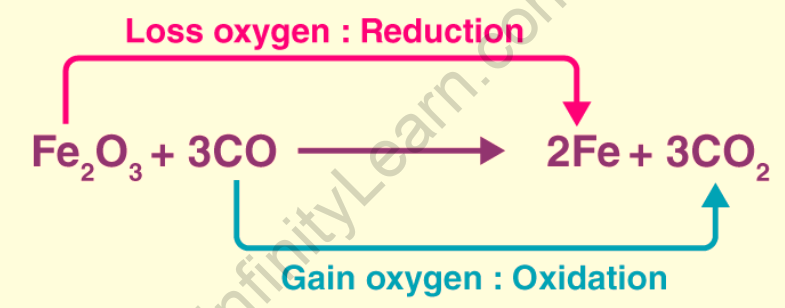
The term oxidation was originally used to describe reactions in which an element combines with oxygen. For example, magnesium oxidation is the chemical reaction that occurs between magnesium metal and oxygen to form magnesium oxide The Latin stem’s “to lead back” sense inspired the word reduction. As a result, in the previously mentioned chemical reaction, everything that leads back to magnesium metal implies reduction. A reaction between magnesium oxide and carbon at 2000 degrees Celsius to form magnesium metal and carbon monoxide is an example of magnesium oxide reduction to magnesium metal.
Overview
An oxidation-reduction reaction is a type of chemical reaction in which electrons are transferred between two elements. It is a chemical reaction in which the oxidation number of an ion, atom, or molecule changes due to the loss or gain of an electron. Redox reactions are at the heart of life’s sustenance, supporting some of the most important chemical reactions such as photosynthesis, respiration, corrosion, and combustion. Oxidation and reduction (redox) reactions are important because they ignite the planet’s natural or biological and artificial energy sources. By removing hydrogen and replacing it with oxygen, molecule oxidation typically produces large amounts of energy. Classical or earlier concepts define oxidation as the addition of oxygen or any electronegative element or removing hydrogen or an electropositive element. Oxidation is defined as the process by which an atom or ion loses one or more electrons in an electronic sense.
Oxidation is a chemical process that can be explained using the four perspectives listed below.
- When it comes to oxygen transfer
- When it comes to electron transfer
- When it comes to hydrogen transfer
- In terms of the number of oxidations,
Oxidation in terms of oxygen transfer – Oxidation is how oxygen is gained.
In terms of electron transfer, oxidation is the loss of electrons. In the preceding example, magnesium loses two electrons and oxidizes to form magnesium oxide.
Oxidation in terms of hydrogen transfer – Oxidation is defined as hydrogen loss.
Oxidation in terms of oxidation number – Oxidation is defined as an increase in an atom’s oxidation state or oxidation number during a reaction. The degree of oxidation of an atom in a chemical compound is defined by the oxidation number.
Redox reaction
The oxidation states of the reactants change in redox reactions, which are oxidation-reduction chemical reactions. The term ‘redox’ refers to the reduction-oxidation process. All redox reactions fall into one of two categories: reduction or oxidation. The redox reaction, also known as the Oxidation-Reduction reaction, always involves simultaneous oxidation and reduction reactions. In a chemical reaction, the substance being reduced is known as the oxidizing agent, while the substance being oxidized is known as the reducing agent.
A redox reaction is a chemical reaction in which electrons are transferred between two reactants that are involved. The change in the oxidation states of the reacting species can be used to identify this electron transfer. The diagram below depicts the electron transfer between two reactants in a redox reaction. The reactant, an electron, was removed from reactant A, and this reactant was oxidized, as shown in the illustration below. Similarly, reactant B was given an electron and thus reduced.
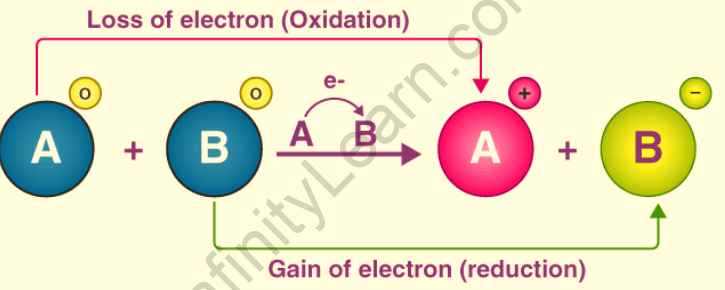
The loss of electrons and the subsequent increase in the oxidation state of a given reactant is characterized as oxidation. The process by which electrons are obtained and the oxidation state of a reactant falls is known as reduction. Oxidizing agents are electron-accepting species that tend to undergo a reduction in redox reactions. A reducing agent is an electron-donating species that tends to give electrons away. Any redox reaction can be divided into two half-reactions, namely the oxidation half-reaction and the reduction half-reaction.
The various types of redox reactions are as follows:
- Decomposition Reaction
- Combination Reaction
- Displacement Reaction
- Disproportionation Reaction
Oxidizing agent
An oxidizing agent (also known as an oxidizer or an oxidant) is a chemical species that tend to oxidize other substances, causing the oxidation state of the substance to increase by causing it to lose electrons. Halogens (such as chlorine and fluorine), oxygen, and hydrogen peroxide are examples of oxidizing agents (H2O2).
Oxidizing agents can be classified into two types:
As an electron acceptor – These are chemical substances in which the atoms remove at least one electron from another atom during a chemical reaction. Oxidizing agents, according to this definition, are the reactants that undergo a reduction in redox reactions. The following diagram depicts the electron-accepting properties of oxidizing agents.
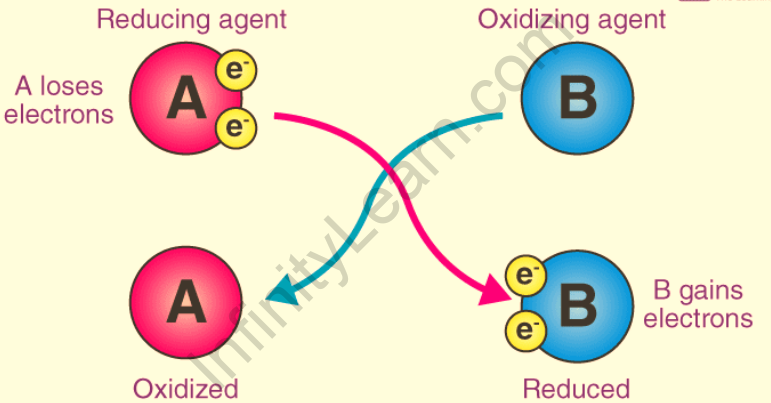
As a substance capable of transferring atoms – In a chemical reaction, an oxidizing agent is a substance that transfers at least one electronegative atom to a chemical species. The transferred atom is an oxygen atom. A transfer of an electronegative atom between two reactants is involved in a number of combustion reactions and organic redox reactions.
Oxidation and reduction
Classical or earlier concepts define oxidation as the addition of oxygen or any electronegative element or the removal of hydrogen or an electropositive element. Oxidation is defined as the process by which an atom or ion loses one or more electrons in an electronic sense. Classical or earlier concepts define reduction as the addition of hydrogen or an electropositive element or the removal of oxygen or any electronegative element. The process by which an atom or ion gains one or more electrons is defined as a reduction in electronic terms.
The addition of oxygen or the more electronegative element to a substance or the removal of hydrogen or the more electropositive element from a substance is called an oxidation reaction, according to another definition of oxidation reactions.
Here are a few examples of oxidation reactions:
2S(s) + O2 (g) → SO2 (g) CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l)
Reduction reactions, like oxidation reactions, are defined as electron gains. Any substance that gains an electron during a chemical reaction undergoes reduction. In other words, the reduction reaction is the addition of hydrogen or a more electropositive element to a substance or the removal of a more electronegative element or oxygen.
2CH2CH2 (g) + H2 (g) → CH3CH3 (g)
Oxidation occurs when a reactant loses electrons during a reaction. Reduction occurs when a reactant accumulates electrons during a reaction. This is a common occurrence when metals react with acid. Oxidation occurs when a reactant loses electrons during a reaction. Reduction occurs when a reactant accumulates electrons during a reaction. This is a common occurrence when metals react with acid. A reduction-oxidation reaction, also known as a redox reaction, is a type of chemical reaction in which reduction and oxidation occur simultaneously. The reduced species gains electrons, while the oxidized species loses them. Despite its name, an oxidation process does not require the presence of oxygen.
FAQs
What exactly is the distinction between oxidation and reduction?
If a substance is oxidized, it loses electrons in a chemical reaction. If a substance is reduced, it gains electrons in a reaction. A REDOX reaction occurs when there is both oxidation and reduction in a reaction.
What is the significance of oxidation and reduction?
The importance of oxidation-reduction (redox) reactions cannot be overstated because they are the primary natural, biological, and artificial energy sources on this planet. By removing hydrogen and replacing it with oxygen, oxidation of molecules typically releases large amounts of energy.
What exactly are reducing agents?
In oxidation-reduction reactions, reducing agents are electron-donating species that readily undergo oxidation. These species tend to lose electrons in redox reactions, increasing their oxidation number. Zinc and lithium are two examples.








