Table of Contents
Energy Bands in Solids: The configuration of molecules in gaseous substances is spread out and not so near to each other. The molecules in liquids are nearer together. However, because the molecules in solids are close together, the atoms of molecules tend to travel into the orbitals of nearby atoms. As an outcome, whenever atoms collide, their electron orbitals overlap.
The blending of atoms in materials causes the development of various energy levels bands. Such groups of energy levels are considered energy bands. Because of the somewhat varying patterns of the surrounding charges, each electron has a variable energy level inside a solid crystal.
The Energy Bands are a continuous energy variation formed by these electron energy levels. When compared to weakly bound electrons, the energy bands of more firmly bound electrons have lower energy (greater negative energy).
Overview of Energy Bands in Solids
Whenever the atoms of a given element are separated by a large distance, each atom has a set of acceptable energy levels. However, when atoms are packed closely together in a solid, the electric fields of surrounding atoms severely disturb the migration of the outer – valence – electrons, changing the precise energy levels displayed in an individual atom.
The extent of the impact of surrounding atoms in a given element is determined by the spacing and placement of the electron within the group of atoms. As a result, when additional atoms are close by, the discrete electron energy levels of single atoms shift into energy bands.
An energy band is a range of energy levels with a large number of allowable adjacent energy levels that are extremely closely spaced.
Origin of Energy Bands Formation in Solids
As per studies, it is found that the electrons in each orbit of an isolated atom have a specific amount of energy. Apparently, in solids, the energy level of the outermost orbit electrons is influenced by the atoms nearby.
The electrons in the outermost orbit encounter an attractive attraction from the nearest or neighboring atomic nucleus when two isolated charges are brought close together. Due to this, electron energies will not be at the same level, and electron energy levels will be modified to a value that is greater or lower than the original energy level of the electron. We can say that the energy levels of electrons in the same orbit may differ.
The phrase “energy band” refers to how these varied energy levels are grouped together. It is found that the energy of inner orbit electrons is unaffected by the existence of nearby atoms.
Formation of Energy Bands in Solids
Different electrons have discrete energies in an isolated atom. If two solitary atoms are brought very near together, the electrons in their orbits will interact with one another. As a result, the energies of electrons in the combined system will not be at the same level but will shift, and the energies will be slightly lower and larger than the original value. As a result, there are two closely spaced energy levels at each energy level’s location.
Bands of permissible energies are formed when the ‘N’ number of atoms are brought together to create a solid and their electrons interact to produce ‘N’ number of closely spaced energy levels in place of discrete energy levels.
There are empty energy zones between the bands of permissible energies, referred to as the banned band of energies. The presence of these bands of energies is supported by the Kronig-Penney model (allowed bands and forbidden bands).
Although the mathematical solution to Schrödinger’s wave equation is laborious, it does provide insight into the genesis of energy bands.
Energy Band Theory and Structure in Solids
Every atom’s shell, according to Bohr’s theory, has a finite amount of energy at various levels. The interaction of electrons between the outermost and innermost shells is explained by the energy band theory. There are three separate energy bands, according to the energy band theory:
(1) Valence Band: Valence electrons are said to be the electrons present in the outermost shell. These electrons produce an energy band known as the valence band, which has a number of energy levels. Here, the occupied energy in the valence band is said to be the highest.
(2) Conduction Band: Because the valence electrons are not strongly bound to the nucleus, a few of them exit the outermost orbit and become free electrons even at ambient temperature. The free electrons are being conduction electrons because they transmit current in conductors. A conduction band has the lowest occupied energy levels and contains conduction electrons.
(3) Forbidden Energy Gap: The prohibited gap is the space between the valence band and the conduction band. The forbidden gap has no energy and no electrons stay in this band, as its name implies. The valence band electrons are securely bound or firmly bonded to the nucleus if the forbidden energy gap is higher. It is needed a particular amount of external energy to fill the restricted energy gap.
Every electron in an atom can only take one of a few allowable orbital configurations with specific energies. While electrons in an atom can only occupy certain energy levels, other neighboring atoms affect the precise energy levels of an individual atom’s electrons in a lattice. As a result, energy levels shift, allowing electrons to flow within certain energy bands. The valence orbitals are the valence electrons’ ground-state orbitals. There are numerous other higher-energy empty crystalline orbitals with bands of permissible energy levels. An electron will be boosted from a valence orbital to a higher excited orbital by an excited crystal. Energy is defined as the difference in energy between the highest valence energy level and the lowest excited orbital energy level.
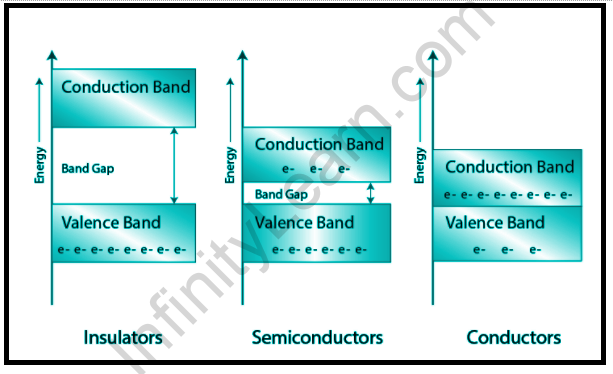
Plotting the available energies for electrons in the materials is a good approach to visualize the differences between conductors, insulators, and semiconductors. Instead of separate energies, as in the case of unbound atoms, the alternative energy states form bands. The conduction process is dependent on the presence of electrons in the conduction band. The electrons in the valence band are separated from the conduction band by a large gap in insulators; the valence band overlaps the conduction band in conductors, such as metals; and the valence and conduction bands are separated by a small sufficient gap in semiconductors that thermal or other pulses can bridge the gap.
The Fermi level, which is the top of the accessible electron energy levels at low temperatures, is an important parameter in band theory. In defining electrical properties, the position of the Fermi level in respect to the conduction band is critical.
FAQs
Why are Band Gaps important?
The use of band gaps to distinguish between conductors, semiconductors, and insulators is very beneficial.
What is a band model?
The existence of energy bands is postulated in band theory to represent the behavior of electrons in materials. It satisfactorily explains several physical features of solids by using the band structure of the material
Does the Band Gap increase with the decrease in the size of the parti
The energy bands are created by the merger of a large number of atoms and molecules' clusters of energy levels. As the particle's size lowers, the number of overlapping orbitals or energy levels drops, narrowing the bands' width and increasing the energy gap between the valence and conduction bands.




