Table of Contents
The physical change of a gas to a liquid form is known as liquefaction (condensation). Gas liquefaction is a complex process that employs a variety of compressions and extensions to reach high pressures and extremely low temperatures, such as turboexpanders. It’s tough to transfer gasses. It is nearly impossible to transport them from one location to another due to their physical features. The gas is changed into a liquid for the same reason. The study of gas liquefaction reveals information about a gas’s properties and structure. It also provides useful information on a matter’s structure in general.
A brief outline
The change of a gas molecule into a liquid condition is known as liquefaction—changes in physical circumstances such as temperature, pressure, and volume cause this transformation. Thomas Andrew is the first to investigate the transition of carbon dioxide from a gas to a liquid state. Most genuine gasses, it was later discovered, behave as Carbon Dioxide (CO2) and shift from gasses to liquids when the right physical adjustments in temperature and pressure are made. Andrews discovered that the gasses could not liquefy at high temperatures, despite great pressure, in his CO2 experiment. The gasses also deviate significantly from their optimum behavior as the temperature rises. In the instance of carbon dioxide, the gas began to change at 30.98° C.
Important concepts
Gas Liquefaction Methods
The liquefaction of gasses can be accomplished in a variety of ways. Among these, the two most critical for air liquefaction are detailed below:
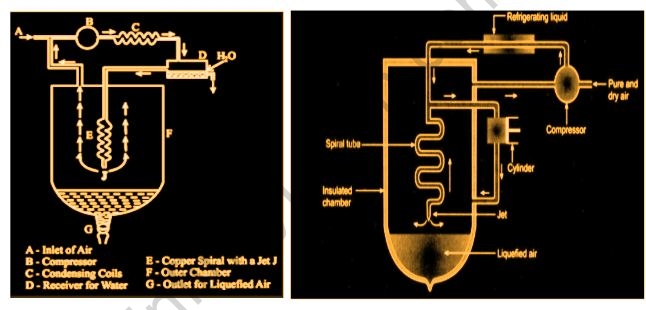
1. Linde’s Approach
The Joule-Thomson effect is used in this procedure. Pure, dry air is taken care of into a blower, which packs it to around 200 atm. The fierceness of pressure is then eliminated by going through a conductor cooled by a refrigerating fluid-like fluid smelling salts. The compressed air is then steered by means of a protected chamber and into a twisting line with a fly toward one side. The packed air extends as it goes through the stream, bringing about a huge decrease in temperature. The extended air ascends through the chamber, cooling the outside air entering the winding cylinder. It would then be gathered and returned to the blower through a channel. At the point when the air is adequately chilled and melted, the technique is performed over and over.
2. Claude’s Approach
This method involves mechanical work and leverages both Joule-Thomson and adiabatic expansion effects of the gas. The blower acknowledges just perfect, dry air, which is compacted to around 200 atm. The hotness of pressure is then taken out by cooling it with a refrigerating fluid. A cylinder ships the compacted gas to a protected chamber. It is isolated into two sections here. One part is held through a twisting cylinder with a stream toward the end, where it goes through the Joule-Thomson extension, and a temperature drop is recorded. The other part is taken to the motor chamber, where it accomplishes mechanical work by pushing back the cylinder and getting cool. It then, at that point, enters the protected chamber and blends in with the air emerging from the fly. It then, at that point, cools the line conveying the approaching air. The cooled air is gathered and taken to the blower once more. The whole interaction is rehashed again and again when the air gets adequately cooled and gets condensed.
Liquefaction of Gasses can be Achieved By:
The cooling of gas can, as a rule, be completed by utilizing the accompanying standards:
- By packing the gas underneath its basic temperature.
- Joule-Thomson impact: When an exceptionally compacted gas, at a temperature underneath its reversal temperature, is gone through a choke (a permeable attachment or stream) from a district of high strain to low tension under adiabatic circumstances, it experiences a fall in temperature. This peculiarity is known as the Joule-Thomson impact and is generally utilized for the liquefaction of gasses.
In the liquefaction of gasses, the temperature required is significant. Just when gas is underneath its basic temperature, it can be condensed. Gasses with a high basic temperature, like NH3, CO2, SO2, and others, can be condensed by adding adequate tension. Gasses with low or exceptionally low basic temperatures, like H2, He, and others, can’t be melted basically by adding strain to them. They can melt if they are chilled underneath their basic temperature and presented to adequate tension.
Liquefaction of helium
Helium is the only liquid gas at temperatures under -259 degrees Celsius, and its inversion point is substantially lower than Hydrogen, at roughly -233 degrees Celsius. Helium has a boiling point of -267 degrees Celsius, near to ground zero on the Kelvin scale. Helium was identified over a century after Hydrogen was discovered, 129 years to be exact. Helium is more difficult to liquefy than other gasses because it is a noble gas. The interatomic forces are weak and have a low atomic mass, bringing it closer to the qualities of an ideal gas than other gasses.
Helium is first compressed to a pressure of 20 atm, raising its temperature to around 300 degrees Kelvin. After that, the compressed high-temperature Helium is divided into two routes. The first is cooled with Helium vapors in the heat exchanger labeled HE1, while the other is cooled with Hydrogen vapors in the heat exchanger labeled HE2. These streams are combined and transferred via the HE3 liquid hydrogen heat exchanger before being cooled by Helium vapors in HE4. Finally, the Joule Thomson effect is triggered by the throttle valve, and Helium is collected in a liquid condition in the Helium separator.
Significance of liquefaction of gasses in NEET exam
Experienced instructors organized Infinity Learn’s NCERT Solution for science. They attempt to isolate any topic into sensible bumps. They began by looking at the essential setting of subjects and explaining the model. Then, they turned out the indispensable requests and answers as students would easily answer on the NEET test. Sentences are a lot made, phonetically sound, and written in a reasonable manner. There is moreover a magnificent opportunity to clear any issue associated with inquiries with the boundlessness to get to know the application. Experts are all available to assist students with their requests.
Also read: Important Topic of Chemistry: Gas law
Frequently Asked Questions
What is the liquefaction cycle?
The liquefaction cycle changes a vaporous substance into a fluid condition. For example, oxygen is typically a vaporous substance that might be changed to a fluid by applying sufficient strain and bringing down the temperature.
What is the advantage behind gas liquefaction?
The main advantage of gas liquefaction is that it permitted gasses to be put away and moved in a considerably more reduced structure than when they were vaporous. LPG and Liquid Oxygen, for instance, are two of the main gasses.
What is the principle behind gas liquefaction?
The principle is that the liquefaction of gasses demands considerable pressure and low temperature. Increasing the pressure leads the gas molecules to move closer together while lowering the temperature leads the attractive forces to rise.









