Table of Contents
An electrophilic substitution reaction is a chemical process in which a compound’s functional group is replaced by an electrophile. Typically, the displaced functional group is a hydrogen atom. Electrophilic substitution reactions are typically carried out in three phases, which are as follows:
- The emergence of an electrophile
- A carbocation’s formation (which is an intermediate)
- The elimination of a proton from an intermediate
The primary distinction between nucleophilic and electrophilic substitution reactions is that nucleophilic substitution reactions require the displacement of a leaving group by a nucleophile, whereas electrophilic substitution reactions involve the displacement of a functional group by an electrophile.
Types of Electrophilic Substitution Reactions
Organic compounds undergo two types of electrophilic substitution reactions: electrophilic aromatic substitution reactions and electrophilic aliphatic substitution reactions.
Electrophilic Aliphatic Substitution Reaction
An electrophile replaces the functional group (usually hydrogen) in an aliphatic molecule in electrophilic aliphatic substitution processes. These reactions can be divided into five categories:
- Ketone halogenation
- Nitrosation
- Tautomerism of keto-enol
- Carbene insertion into a carbon-hydrogen bond
- Diazonium fusion (aliphatic)
These electrophilic substitution reactions might result in configuration inversion if the electrophilic attack occurs at a 180-degree angle to the leaving group (attack from the rear).
Electrophilic Aromatic Substitution Reaction
Despite having many double bonds, aromatic compounds do not undergo addition reactions. Their lack of reactivity to addition processes is related to the high stability of the ring systems produced by full electron delocalization (resonance). Aromatic compounds react via electrophilic aromatic substitution reactions, which retain the aromaticity of the ring system. Benzene, for example, interacts with bromine to generate bromobenzene.
An atom linked to an aromatic ring is replaced with an electrophile in electrophilic aromatic substitution processes. Aromatic nitration, aromatic sulphonation, and Friedel-Crafts reactions are examples of such reactions.
It is crucial to highlight that in electrophilic aromatic replacements, the aromaticity of the aromatic component is conserved. As a result, aromatic rings and iodine, bromine, or chlorine can be employed in these processes to produce aryl halides.
Mechanism Of Electrophilic Substitution
Step 1: Generation of an Electrophile
Anhydrous aluminum chloride is a particularly useful Lewis acid in the formation of electrophiles from the chlorination, alkylation, and acylation of an aromatic ring. The presence of Lewis acid causes the formation of electrophiles. The Lewis acid accepts the electron pair from the attacking reagent. The electrophiles formed are Cl+, R+, and RC+O, in that order (from the combination of anhydrous aluminum chloride and the attacking reagent).
Step 2: Formation of carbocation
The electrophile targets the aromatic ring by producing a sigma complex or an arenium ion. sp3 is one of the hybridized carbons in this uranium ion. This arenium ion finds stability in a resonance configuration. Because electron delocalization ends at the sp3 hybridized carbon, the sigma complex or arenium ion loses its aromatic property.
Step 3: Deprotonation
Finally, the proton is liberated from the arenium or sigma ion complex. This occurs when a reagent like AlCl4 hits the sp3 hybridized carbon. As a result, the aromatic nature of the molecule is restored, and the electrophile eventually replaces the aromatic component’s hydrogen.
Many electrophiles are not electrophilic enough to react independently, or the reaction is protracted. With the aid of Lewis acids, we may additionally speed up the electrophilic substitution process. For example, in the presence of AlCl3 and FeCl3, the electrophilic substitution process of benzene with Cl2 can proceed at a substantially higher pace. We now know that when alcohol groups undergo substitution or elimination processes, they generate conjugate acid.
However, if we use a strong acid like HCl to convert the alcohol into its conjugate acid R-OH2+, the process can occur considerably quicker. This is due to Cl– replacing the far weaker base H2O. As a result of the H+ weakening the C-O bond, the carbon is connected to the electrophile considerably more effectively. The identical process is used to Lewis acids introduced from the electrophile in electrophilic substitution reactions. This weakens the electrophilic molecule’s link, making it an even greater nucleophile compound.
Examples of Electrophilic Substitution Reaction
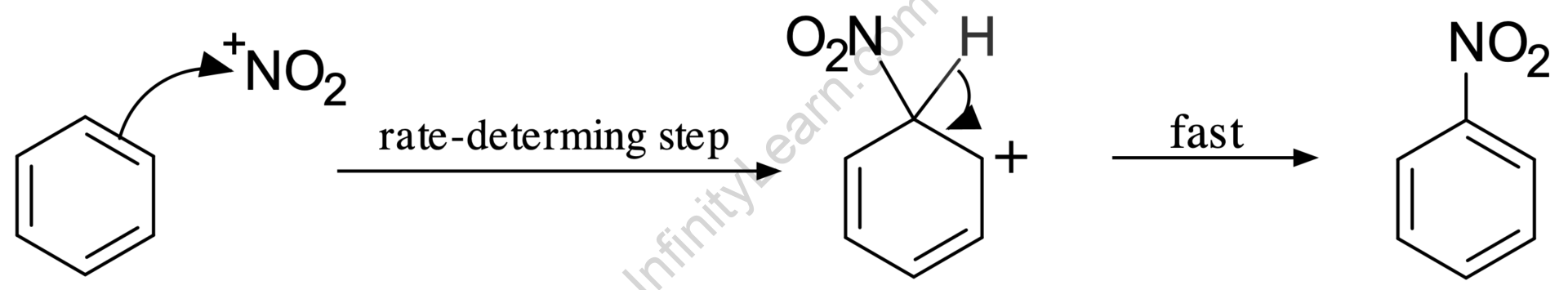
Nitration and halogenation of benzene are two instances of electrophilic aromatic substitution. The electrophiles are nitronium ion (NO2+) and sulfur trioxide (SO3), which combine with benzene to form nitrobenzene and benzene sulfonic acid, respectively.
- Sulfonation of Benzene
Benzene sulfonation is the process of heating benzene with sulphuric acid (H2SO4+SO3) to produce benzene-sulfonic acid. The reaction is reversible in nature.
- Nitration of Benzene
The source of the nitronium ion induces the loss of a water molecule and the formation of a nitronium ion via protonation of nitric acid by sulfuric acid.
- Halogenation of Benzene
Benzene interacts with halogens to generate aryl halides in the presence of Lewis acid, such as FeCl3, FeBr3. This is referred to as benzene halogenation.
Benzene Electrophilic Substitution
The benzene ring’s resonance causes the delocalized electron to span efficiently over the carbon atoms of the benzene ring. It also helps to stabilize the arenium ion. Because of the partial stability of arenium ions, benzene is especially prone to electrophilic substitution reactions.
The electrophilic substitution of benzene occurs when an electrophile replaces the hydrogen atom of benzene. Because the aromaticity of benzene is not altered throughout the reaction, these reactions are very spontaneous. Basic benzene electrophilic substitution reactions include nitration, sulfonation, halogenation, Friedel Craft’s alkylation, acylation, and so on.
Also read: Important Topic of Chemistry: Ozonolysis
FAQs
What are the Catalysts Used in Aromatic Ring Chlorination and Bromination?
Lewis acid catalysts like AlCl3 and FeCl3 significantly accelerate the chlorination of an aromatic ring. Because the Lewis acids form a complex with the chlorine molecule, a highly electrophilic Cl+ species is formed. Catalysts such as AlBr3 and FeBr3 can be employed instead of brominating an aromatic ring.
What are the key distinctions between the electrophilic substitution reaction and the nucleophilic substitution reaction?
Both nucleophilic and electrophilic substitution reactions are seen in organic and inorganic chemistry. Electrophilic substitutions involve the displacement of a functional group by an electrophile (generally a hydrogen atom). The assault of a nucleophile on a positively charged (or partly positively charged) atom or community is an example of a nucleophilic replacement.







