Table of Contents
The inductive effect, also known as “the -I Effect,” is a distance-dependent phenomena in which the charge of a chemical bond influences the orientation of nearby bonds in a molecule, resulting in a permanent state of polarization.
How Does It Work?
When atoms from two distinct elements engage in a link, the electron density is not uniform. In a bond, electron clouds prefer to arrange themselves toward the bond’s more electronegative element.
In water molecules, the inductive effect occurs. Near the hydrogen atoms, the chemical bonds within a water molecule are more positively charged, whereas near the oxygen atoms, they are more negatively charged. Water molecules are hence polar. However, because the induced charge is modest and the inductive effect is only active over short distances, it can be easily negated by other variables.
Acidity and Basicity, as well as the inductive effect
In chemistry, the terms “acidity” and “basicity” describe the tendency of a chemical species to donate or accept protons, respectively. The strength of an acid or base is usually measured by its ability to dissociate in water. The more easily a molecule can donate or accept protons, the stronger the acid or base.
The inductive effect is a chemical property that describes the way that a molecule’s electron density can be shifted by the presence of another molecule. The inductive effect can cause an acid or base to become stronger or weaker, depending on the nature of the molecule.
Resonance
The formation of several Lewis structures within a molecule as a result of a double bond generated with equal probability between distinct atoms is known as resonance.
Ozone (O3), for example, has resonance forms. Because single bonds are normally weaker/longer than double bonds, one would ask if the bond created between two oxygen atoms has different length.
Because resonance forms (drawn on paper) don’t represent what’s going on within the molecule (it doesn’t have a double bond and a single bond), each bond is the same length and strength in reality. Instead, electrons are uniformly distributed across the atoms, generating intermediate bonds between single and double bonds.
C-C-C-Y
The Y-atom is less electronegative than the C-atom in this case.
C attracts -electrons to itself, thus a partial negative charge shows on carbon as -, while a positive charge appears on the Y-atom as +.
Because it gives electrons to Carbon, Y is an electron-donating group or + I group.
This phenomenon is also known as the electron-donating inductive effect or the positive inductive effect (+ I effect).
Note that the inductive effect only takes into account the partial shifting of -electrons or a single bond.
FAQs
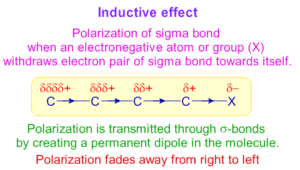
Q. What is -I Effect (Negative Inductive Effect) ?
Ans: When an electronegative element, such as a halogen, is added to a chain of atoms (usually carbon atoms), the uneven distribution of electrons produces a positive charge that is carried along the chain.
The resulting effect is known as the electron-withdrawing inductive effect, or the -I effect, since it generates a permanent dipole in the molecule where the electronegative atom has a negative charge.
Q. How can you determine the acidity of organic and unsaturated compounds?
Ans: Remove the proton from an organic molecule to determine its acidity, and then assess the stability of the resultant conjugate base. The stronger the acid, the more stable the conjugate base is.
Check the hybridization of the carbon involved to see if an unsaturated molecule is acidic. The higher the s-character on a carbon, the higher its electronegativity, and therefore the acidity.
As a result, the most acidic alkynes, alkenes, and alkanes are: Alkynes > Alkenes > Alkanes Alkynes > Alkenes Alkanes Alkanes Alkanes
What is +I Effect (Positive Inductive Effect) ?
The charge is relayed across a carbon chain when a chemical species with the potential to release or donate electrons, such as an alkyl group, is added, and this process is known as the Positive Inductive Effect or the +I Effect.