Table of Contents
Mix aniline in hydrochloric acid and add acetic anhydride, stirring it well. Pour the sodium acetate mixture into water. You’ll get acetanilide, which can be separated and purified using ethyl alcohol.
Also Check: CBSE syllabus 2024
To Prepare a Sample of Acetanilide from Aniline
Theory
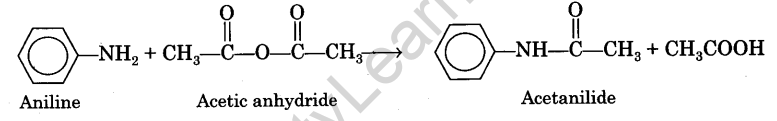
Acetanilide is prepared by acetylating aniline with acetic anhydride in the presence of glacial acetic acid. The chemical equation can be written as :
Apparatus
Round bottom flask (100 ml), water condenser, wire-gauze, tripod stand, burner, iron-stand, clamp, measuring cylinder, etc.
Chemicals Required
Aniline = 5 ml
Acetic anhydride = 5 ml
Glacial acetic acid = 5 ml.
Procedure
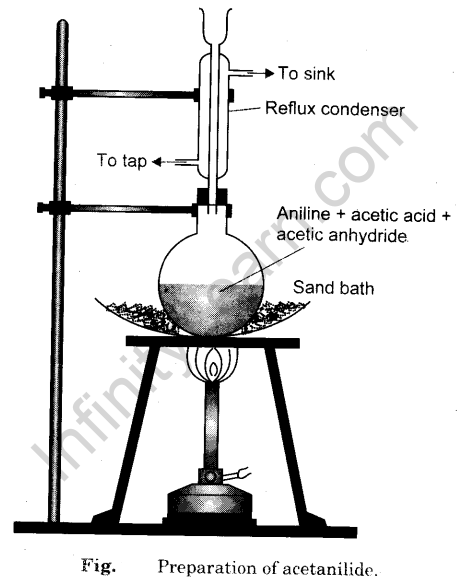
- Take 5 ml of acetic anhydride in a clean dry 100 ml conical flask and add 5 ml of glacial acetic acid and shake the contents thoroughly.
- To this mixture taken in the flask, add 5 ml of aniline and fit a water condenser.
- Place the flask on a wire-gauze placed on a tripod stand as shown in Fig.
- Boil the mixture for 10-15 minutes.
- Allow the mixture to cool. Detach the condenser and pour the liquid into about 150 ml ice-cold water contained in a beaker. During addition, stir vigorously the contents of the beaker with the help of glass-rod.
- Filter the white precipitates which separate out and wash with cold water.
- Recrystallise from hot water containing a few drops of ethyl alcohol. Weigh the crystals and record the yield.
- Determine the melting point of the compound.
Result
Weight of acetanilide obtained =…………g
Melting point of acetanilide = ……..°C
Note: Acetanilide has white flaky crystals. Its melting point in 113°C.
Precautions
- Freshly distilled aniline should be used in order to get good results or small amount of zinc can be added in the reaction mixture. Zinc reduces the coloured impurities in the aniline and also prevents its oxidation during the reaction.
- Prolonged heating and use of excess of acetic anhydride should be avoided.
- Reaction mixture should first be cooled and then poured in ice-cold water otherwise hydrolysis of acetanilide may take place.
Preparation of Acetanilide FAQs
How do you separate acetanilide from aniline?
Mix the acetic anhydride with the aniline hydrochloride in water. Swirl it together and quickly add the sodium acetate solution. The mix turns white as acetanilide forms. Chill the mix in an ice bath and gather the solid acetanilide using a special filter.
How is acetanilide prepared Class 11?
Acetanilide is made by heating acetic anhydride or glacial acetic acid with aniline in the presence of zinc dust. This reaction forms acetanilide and acetic acid. Zinc dust is used to stop oxidation during the process.
What is the percentage yield of acetanilide from aniline?
Percent yield of acetanilide is 75.86%.
What is the color of acetanilide?
Acetanilide can somewhat dissolve in water and stays unchanged in most situations. When it forms crystals, they look like flat plates and can be colorless, white, or something in between.




