Download Solved CBSE Sample Papers for Class 9 Science Set 3 2019 PDF to understand the pattern of questions asks in the board exam. Know about the important topics and questions to be prepared for CBSE Class 9 Science board exam and Score More marks. Here we have given Science Sample Paper for Class 9 Solved Set 3.
Board – Central Board of Secondary Education, cbse.nic.in
Subject – CBSE Class 9 Science
Year of Examination – 2019.
Solved CBSE Sample Papers for Class 9 Science Set 3
Time Allowed: 3 hours
(GENERAL INSTRUCTIONS)
(i) The question paper comprises of two sections. A and 13. You are to attempt both the sections.
(ii) All questions are compulsory. However, internal choice has been provided in two questions of three marks each and one. question office marks. Only one option in. such questions is to be attempted.
(iii) All questions of section A and all questions of section B are to be attempted separately.
(iv) Question numbers 1 and 2 in section A are one mark questions. These are to be answered in one word or in one sentence.
(v) Question numbers 3 to 5 in section A are two marks questions. These are to be answered in about 30 words each.,
(vi) Question numbers 6 to 15 in section A are three marks questions. These are to be answered in about 50 words each..
(vii) Question numbers 16 to 21 in section A are five marks questions. These arc to be answered in about 70 words each.
(viii) Question numbers 22 to 27 in Section B are two marks questions based on practical skills. These are to he answered in brief.
SECTION – A
Question 1:
Write the units of ‘g’ and ‘G’.
Answer:
SI unit of ‘g’ is m s-2 and that of ‘G’ is N m2 kg-2.
Question 2:
J. Chadwick discovered a subatomic particle which has no charge and has mass nearly equal to that of a proton. Name the particle and give its location in the atom. Answer:
The particle is called neutron. It is located in the nucleus of the atom.
Question 3:
You are provided with a fine white coloured powder which is either sugar or salt. How will you identify it without tasting ?
Answer:
Salt is NaCI which is an ionic compound. Its solution reacts with a solution of silver nitrate (AgNO3) which is also ionic in nature, to give a precipitate of silver chloride. On the other hand, sugar is not ionic in nature and does not give this type of reaction. So, if we obtain a white precipitate on adding silver nitrate solution to the solution of the given substance, it is salt otherwise it is sugar.
Question 4:
Why do some children fall ill more frequently than others living in the same locality ? Answer:
Children living in same locality implies that they are exposed to same surroundings. However, some fall ill more frequently. This could be because they may be under-nourished which may result in poor immunity and hence their catching infections more easily.
Also personal hygiene, genetic make up etc., play a vital role.
Question 5:
“Every multicellular organism has arisen from a single cell.” Justify this statement.
Answer:
Multicellular organisms are higher organisms and usually undergo sexual reproduction in which there is fusion of gametes from two parents involved. This is called fertilisation and results in formation of a single cell called zygote which undergoes cell division and differentiation to form a multicellular organism.
Question 6:
(a) A person moves a distance of 3 km towards east, then 2 km towards north and then 3.5 km towards east. Find :
(i) the distance covered by the person,
(ii) the displacement of this motion.
(b) Name the type of motion in which a body has uniform speed but not uniform velocity ?
Answer:
(a) Distances of AB = 3 km east, BC = 2 km north and CD = 3.5 km east are shown in figure.

(b) Uniform circular motion.
Question 7:
(a) A block of mass m raised from position A to 6 by taking two different paths as shown in figure (a) and (b). Let the height AB = h. Now answer the following questions:
(i) What is the work done on the block in figure (a) and (6)?
(ii) Name the energy possessed by the block at position B in both the cases. A
(b) Find the potential energy possessed by an object of mass 6 kg when it is at a height of 15 m above the ground.
(Take g = 9.8 m s-2)

Answer:
(a) (i) Work done on the block in both cases is W = mgh.
(ii) At position B the block possesses gravitational potential energy Ep = mgh.
(b) Here mass m = 6 kq, heiqht h = 15 m and q = 9.8 m s-2 Potential enerqy of object
Ep = mgh = 6 x 9.8 x 15 = 882 J
Question 8:
(a) A cork piece floats on water but an iron nail sinks in water. Why ?
(b) What is the difference between density of a substance and its relative density ? Write SI unit of both.
Answer:
(a) A cork piece floats on water because its density is less than the density of water. On the other hand, an iron nail sinks because density of iron nail is greater than the density of water.
(b)
|
Density |
Relative density |
|
(1) The density of a substance is defined as its mass per unit volume, (2) The SI unit of density is kg/m³. |
(1) Relative density of a substance is the ratio of its density to the density of water, (2) Relative density is a unitless quantity. |
OR
Two objects of masses mi and m2, when separated by a distance d, exerts a force F on each other. What happens when
(i) value of mass of first object is doubled ?
(ii) masses of both objects are doubled ?
(iii) masses are brought closer so that distance between them becomes d/2 ?
(iv) the space between the two objects has no air and it is complete vacuum ?
Answer:

Question 9:
(a) What do the graphs of figure indicate ?

(b) On a 120 km track a train travels the first 30 km at a uniform speed of 30 km hr1. Calculate the speed with which the train should move rest of the track so as to get the average speed of 60 km hr1 for the entire trip.
Answer:
(a) Graph of figure
(i) indicates uniformly retarded motion but graph of figure
(ii) shows non-uniformly accelerated motion.

Question 10:
State any four postulates of Dalton’s atomic theory of matter. Which of his postulates does not hold correct at present ?
Answer:
The postulates of Dalton’s atomic theory of matter are :
(i) All matter is made of very tiny particles called atom.
(ii) Atoms of a given element are identical in mass and chemical properties.
(iii) Atoms of different elements have different masses and chemical properties.
(iv) Atoms combine in the ratio of simple whole numbers to form compounds.
(v) Atoms are indivisible particles which cannot be created or destroyed in a chemical reaction.
The postulate that atoms are indivisible particles does not hold good at present. We know that an atom is made up of protons, neutrons and electrons.
Question 11:
Write the electronic configuration and valency of the following:
(i) Chlorine
(ii) Sodium and
(iii) Silicon
Answer:

OR
(a) Both helium and beryllium have Helium is a noble gas whereas Beryllium is a metal, justify.
(b) Hydrogen exists in three isotopic forms. Why are the isotopes of hydrogen chemically alike ?
Answer:
(a) Maximum capacity of the first orbit is 2 electrons. Therefore, helium having two electrons in the valence shell is stable and hence has no tendency to react.
Beryllium has two shells K and L. The maximum capacity of the L-shell is 8 whereas beryllium has only 2 electrons in the L-shell. It will therefore react with other beryllium atoms to form Be-Be bonds. It is therefore a metal.
(b) The three isotopes of hydrogen are ¹H1, ²H1 and ³H1. The chemical property of an element depends upon the number of protons. Since the number of protons in the isotopes is the same, the isotopes are chemically alike.
Question 12:
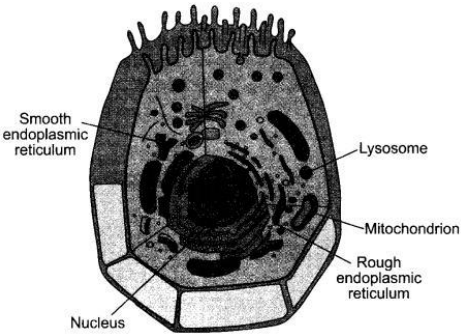
Draw the diagram of an animal cell and label the following :
(i) Factory of ribsomes.
(ii) Power house of cells.
(iii) Waste disposal system of the cell.
(iv) Director/brain/control centre of the cell.
Answer:
(i) Endoplasmic Reticulum
(ii) Mitochondria
(iii) Lysosomes
Question 13:
How are phanerogams classified ?
Answer:
Phanerogams are seed producing/flowering plants. They are classified as :

(i) The seeds of gymnosperms are naked whereas angiosperms bear seeds inside the fruits.
(ii) The sporophylls in gymnosperms are aggregated to form cones. On the other hand sporophylls in angiosperms are aggregated to form flowers.
Question 14:
(a) Name any one disease caused by bacteria and virus.
(b) Why are antibiotics effective against bacteria but not viruses ?
Answer:
(a) Disease caused by –
(i) Bacteria – Tuberculosis
(ii) Virus – AIDS (Acquired Immuno-Deficiency Syndrome)
(b) Antibiotics act by preventing one of the bio-chemical events towards formation of a new pathogen cell. Thus, most prevent cell wall formation, which happens in bacterial cell only, thereby preventing bacterial diseases.
Question 15:
Rameshwar took the responsibility of his fields when his father got old. His father advised him to use farmyard manure over fertilisers but he wanted to use chemical fertilisers. His father told him about the adverse effects of chemical fertilisers to the nearby water bodies.
Rameshwar’s father encouraged him as his friends also use organic manure and careful and judicious use of chemical fertilisers.
(i) Why should chemical fertilisers be used carefully and judiciously ?
(ii) Manures are natural fertilisers. How can they be prepared in the Held ?
(iii) Why did Rameshwar’s father advise him ?
Answer:
(i) Fertilisers should be applied carefully in terms of proper dose, time and observe pre and post application precautions for their complete utilisation. Continuous use of fertilisers in an area can destroy soil fertility because organic matter in the soil is not replenished and micro-organisms in the soil are harmed. Excess use of fertilisers can also lead to water pollution.
(ii) Manure is prepared by the decomposition of plant waste and animal excreta (cowdung). It helps in enriching soil with nutrients and organic matter. It improves the soil structure, increases water-holding capacity and avoids water logging. Based on kind of biological material used, manure can be broadly classified into compost or vermicompost and green manure.
(iii) Rameshwar’s father advised him so as he was concerned about the environment. He cares about other living beings and is a conscientious human being.
Question 16:
(a) Give three medical uses of ultrasound.
(b) A ship which is stationary is at a distance of 2800 m from the sea bed. This ship sends an ultrasound singal to the seabed and its echo is heard after 4 s. Find the speed of sound in water.
Answer:
(a) Important uses of ultrasound in medical field are as follows :
(i) In a ultrasound scanner the ultrasonic waves are used for getting images of internal organs of the body such as liver, gall bladder, kidney, uterus etc. The technique is known as ultra sonography.
(ii) Echo cardiography is a medical diagnostic technique in which ultrasonic waves are used to construct the image of heart.
(iii) Powerful ultrasounds are used for treatment of kidney stones.
(b) Distance of ship from seabed d = 2800 m, time of echo t = 4 s. If speed of sound in water be v, then
![]()
Question 17:
Two objects A andfi, having masses 100 kg and 75 kg, moving with velocities 40 km/h and 60 km/h respectively. Answer the following :
(a) Which will have greater inertia ?
(b) Which will have greater momentum ?
(c) Which will stop first if equal negative acceleration is applied on both ?
(d) Which will travel greater distance in 10 minutes ?
(e) Which will impart greater force if collided with a wall ?
Answer:

’a’, SA may be less than or greater than SB.
(e) The force imparted on collision with a ball is equal to rate of change of momentum of the given object. As a result, object B will impart a greater force.
Question 18:
(i) Account for the following :
(a) The temperature of water remains constant during boiling.
(b) Evaporation is a surface phenomenon.
(c) The shape between the constituent particles is maximum in gases.
(ii) Suppose you want to convert a gas into a liquid, which two methods can you apply ?
Answer:
(i) (a) After water has started boiling, the heat supplied is used to increase the kinetic energy of the particles to convert into gaseous state. Therefore the temperature does not rise.
(b) Particles on the surface absorb energy from the surrounding and escape. Therefore
evaporation is a surface phenomenon.
(c) Particles in a gas have maximum kinetic energy. They are moving freely in all directions and thus space between particles is maximum in gases.
(ii) (a) by increasing pressure.
(b) by lowering the temperature.
Question 19:
Describe any three properties of colloids. Categorise the following examples of colloids into different categories of colloids : jelly, fog, milk, shaving cream.
Answer:
Properties of colloids :
(i) The size of particles of a colloid is too small to be seen individually by naked eyes.
(ii) Colloids are big enough to scatter a beam of light passing through it and makes its path visible.
(iii) They do not settle down when left undisturbed, that is, a colloid is quite stable.

Question 20:
Describe the oxygen-cycle in nature.
Answer:
Oxygen-cycle : Oxygen from the atmosphere is used up in combustion, respiration and in the formation of oxides of nitrogen. It is returned to the atmosphere through photosynthesis.

Question 21:
(a) Give two characteristics of the following phylum :
(i) Platyhelminthes
(ii) Arthropoda
(iii) Porifera.
(b) What is notochord and explain its function ?
Answer:
(a) (i) Platyhelminthes – A phylum of flatworms where the body is dorsoventrally flattened, triploblastic but acoelomate with organ level of organization and bilateral symmetry. They possess soft, elongated leaf like or tape like body which exhibits bilateral symmetry.
(ii) Arthropoda – A phylum of triploblastic, bilaterally symmetrical segmented animals having jointed legs and appendages a chitinous exoskeleton and a blood filled body
cavity called haemocoel. It is the largest group of animals, which are found in variety of habitats like land, moist soil, fresh water, marine water as well as on the bodies of other animals.
(iii) Porifera – Porifera is a phylum of diploblastic, acoelomate animals having cellular level organisation, porous body with canal system and sedentary habit. The body has a covering of hard skeleton which comprises of spicules of calcium carbonate, silica or spongin fibres.
(b) A notochord is a solid rod present in chordates at some stage.
It provides place for muscles to attach. It increases internal support and locomotory power.
OR
(a) List three characteristic features of kingdom Monera.
(b) Write two examples each :
(i) Egg laying mammals
(ii) Organisms with open circulatory system
Answer:
(a) The identifying features of kingdom Monera are as follows :
(i) They are prokaryotes. Membrane bound cell organelles are absent. They have naked genetic material called nucleoid.
(ii) Monerans are basically unicellular. In filaments and colonies the cells are similar and independent.
(iii) Both autotrophic and heterotrophic mode of nutrition are found.
(iv) This kingdom includes bacteria, blue green algae or cyanobacteria and mycoplasma.
(v) Some representatives of this kingdom have a cell wall and some do not have it.
(b) (i) Egg laying mammals – Echidna, Duck billed platypus
(ii) Organisms with open circulatory system – Phylum Arthropoda and Mollusca.
SECTION – B
Question 22:
State the factors on which buoyant force acting on an object immersed in a fluid depend.
Answer:
The magnitude of buoyant force acting on an object immersed in a fluid depends on
(a) the volume of the immersed part of the object, (b) the density of the fluid, and (c) the value of acceleration due to gravity at the given place.
Question 23:
How is experiment of reflection of sound different from the experiment on laws of reflection of light ?
Answer:
For reflection of light we require a polished mirror as the reflecting surface and mark pins to know the direction of incident and reflected rays.
However, for reflection of sound we require a large sized, smooth and hard wooden/metal sheet/wall as the reflecting surface and two long identical plastic pipes of small diameter for passage of incident and reflected sound.
Question 24:
Which portion of temperature-time graph showing heating of ice at – 10 °C to water at 100 °C represent the change of state on heating ?

Answer:
The portion BC represents the heating of ice at 0 °C to water at 0 °C and the portion BE represents of heating of water at 0 °C to 100 °C.
Question 25:
An experiment is being carried out as shown in the figure. Mass of the conical flask with the contents as shown was taken. The flask was shaken so as to allow a reaction between the contents of the ignition tube and the flask. After allowing to come to room temperature, mass of the flask with its contents was taken.
(a) Do you find any difference between the mass before and after shaking ?
(b) Which law of science does this experiment prove ?

Answer:
(a) There is no difference in the mass of the flask before and after shaking.
(b) This experiment proves the law of conservation of mass.
Question 26:
Define venation. Give two types of venation alongwith suitable example.
Answer:
Venation is defined as arrangement of veins (vascular bundles) in the leaves.
It is of two types :
(i) Reticulate – where veins form a network, e.g., mango, peepal tree.
(ii) Parallel – where veins run parallel to each other, e.g., grasses, rice, wheat etc.
Question 27:
Name the phyla to which following organisms belong. Arrange in ascending order of complexity of body design and give a common feature. Moss, Fern, Mucor, Spirogyra
Answer:
Moss – Phylum Bryophyta
Fern – Phylum Pteridophyta
Mucor – Phylum Fungi
Spirogyra – Phylum Thallophyta
Ascending order of complexity of body design :
Mucor —> Spirogyra -» Moss —> Fern
Common feature – All these possess cell wall.
We hope the Solved CBSE Sample Papers for Class 9 Science Set 3, help you. If you have any query regarding CBSE Sample Papers for Class 9 ScienceSolved Set 3, drop a comment below and we will get back to you at the earliest.





