Table of Contents
Matter in Our Surroundings Class 9 Extra Questions Science Chapter 1
Class 9 Science chapter 1 Matter in Our Surroundings in NCERT textbook introduces students to the fundamental concept of matter, which encompasses everything that occupies space and has mass. This chapter explores the physical nature of matter, including its various states—solid, liquid, and gas—and the properties that define them. It also get into important phenomena such as diffusion, evaporation, and the impact of temperature and pressure on matter which are the important topics included in CBSE syllabus. While the chapter provides a comprehensive overview of these concepts, class 9 science chapter 1 extra questions and answers are an invaluable tool for deepening students understanding and mastery of the material.
Extra Questions for Class 9 Science Chapter 1 Matter in Our Surroundings
Below are the long and short type class 9 Science chapter 1 matter in our surroundings extra questions with answers:
Matter in Our Surroundings Class 9 Extra Questions Very Short Answer Questions
Question 1: Is there any similarity in materials?
Answer: Yes, all materials have mass and occupy space, meaning they all have physical presence and weight.
Question 2: When 50 g of sugar is dissolved in 100 mL of water, there is no increase in volume. What characteristic of matter is illustrated by this observation?
Answer: This illustrates that the particles of water have spaces between them, allowing sugar particles to fit in without increasing the overall volume.
Question 3: What happens when an inflated air balloon is pricked with a pin? Name the property of the gaseous state exhibited by this observation.
Answer: When an inflated balloon is pricked, it bursts and the gas inside quickly spreads out, demonstrating the property of diffusion in gases.
Question 4: Name the process which occurs when a drop of Dettol is added to water.
Answer: Diffusion occurs when Dettol is added to water, spreading throughout the water.
Question 5: To which physical state of matter do the following statements apply?
- (i) Incompressible, no fixed shape
- (ii) Compressible, no definite volume
Answer:
- (i) Liquid: Incompressible and takes the shape of its container but has no fixed shape.
- (ii) Gas: Compressible and does not have a definite volume.
Question 6: Name the state of matter in which:
- (i) Layers of particles can slip and slide over one another easily.
- (ii) Particles just move around randomly because of very weak force of attraction.
Answer:
- (i) Liquid state: Particles can slide past each other easily.
- (ii) Gaseous state: Particles move randomly due to very weak forces of attraction.

Question 7: Define density and give its SI unit.
Answer: Density is defined as the mass per unit volume of a substance. Its SI unit is kilograms per cubic meter (kg/m³).
Question 8: In which of the following do the particles have the highest forces of attraction? Water, NaCl (solid), ice, or wax.
Answer: NaCl (solid) has particles with the highest forces of attraction due to its strong ionic bonds.
Question 9: Why do gases exert more pressure on the walls of the container than solids?
Answer: Gases exert more pressure because their particles move rapidly and collide with the walls of the container frequently and with high energy, unlike solids where particles are in fixed positions.
Question 10: Which of the following diffuses faster? Water vapour, wax, or ethyl alcohol.
Answer: Water vapour diffuses faster because it is in the gaseous state, allowing its particles to move more freely and quickly.
Question 11: Why do we see water droplets on the outer surface of a glass containing ice-cold water?
Answer: Water vapour from the air condenses into liquid droplets when it comes into contact with the cold surface of the glass.
Question 12: Can materials exist in all three states?
Answer: Yes, materials can exist in solid, liquid, and gaseous states under different conditions of temperature and pressure.
Question 13: Kinetic energy of particles of water in three vessels A, B, and C are EA, EB, and EC respectively and EA > EB > EC. Arrange the temperatures, TA, TB, and TC of water in the three vessels in increasing order.
Answer: The order of temperatures is TC < TB < TA, indicating that higher kinetic energy corresponds to higher temperature.
Question 14: Analyse the temperature versus time graph of water given below.
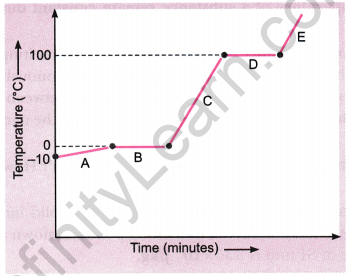
Which region contains all liquids?
Answer: Region C contains all liquids
Matter in Our Surroundings Class 9 Extra Questions Short Answer Questions-I
Question 1: When a crystal of potassium permanganate is placed in a beaker of water, its purple color spreads throughout. What does this observation conclude about potassium permanganate and water?
Answer: The purple color spreading throughout the water indicates diffusion. Potassium permanganate, being a solid, initially settles at the bottom, but water molecules collide with it and the particles intermingle due to sufficient space between the molecules in the liquid state.
Question 2: Why do solids have a regular geometrical shape?
Answer: Solids have a regular geometrical shape because their particles are arranged in a highly ordered structure due to strong intermolecular forces.
Question 3:Why are gases compressible but not liquids?
Answer: Gases are compressible because the intermolecular spaces in gases are large, allowing particles to be pushed closer together. In liquids, the intermolecular spaces are smaller, making them incompressible.
Question 4: Can a rubber band change its shape on stretching? Is it a solid?
Answer: Yes, a rubber band changes shape when stretched and regains its shape when the force is removed. It is considered a solid because it maintains its shape unless excessive force is applied.
Question 5: Why is steam at 100°C better for heating purposes than water at 100°C?
Answer: Steam at 100°C is better for heating because it contains additional energy from the latent heat of vaporization, making it more effective than water at the same temperature.
Question 6: Give two ways in which melting points and boiling points are useful.
Answer:
- They help in checking the purity of a substance.
- They assist in identifying and characterizing substances.
Question 7: Alka felt intense heat from steam while making tea. Is the temperature of steam higher than boiling water?
Answer: No, the temperature of both steam and boiling water is 100°C. However, steam has more energy due to the latent heat of vaporization.
Question 8: Why does the temperature of a substance remain constant during its melting or boiling point?
Answer: The temperature remains constant during melting or boiling because the heat supplied is used to change the state by overcoming the forces of attraction between particles, a process that involves latent heat.
Question 9: What is the latent heat of fusion? What is the latent heat of fusion of ice?
Answer: Latent heat of fusion is the heat required to convert 1 kg of solid into liquid at its melting point without changing the temperature. For ice, it is 3.35 × 10^5 J/kg.
Question 10: Which gas is called dry ice and why?
Answer: Solid CO2 is called dry ice because it sublimates directly into gas without passing through the liquid state when the pressure is decreased to 1 atmosphere.
Question 11: If a glass tumbler of hot water is kept in a freezer, which graph represents its temperature change over time?
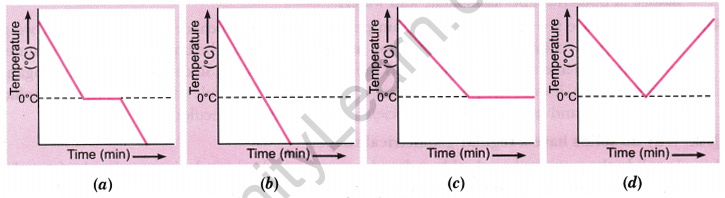
Answer:
(a) Initially, the water cools until it reaches 0°C, the freezing point. At this stage, the temperature remains constant as the water freezes. Once all the water has turned into ice, the temperature will drop further.
Question 12: Why do doctors advise putting strips of wet cloth on the forehead of a person with high fever?
Answer: Doctors recommend placing wet cloth strips on the forehead of someone with high fever because the water in the cloth evaporates, absorbing heat from the body. This evaporation process helps to lower the body’s temperature, providing relief from the fever.
Question 13: Look at the following figures and suggest in which of the glass containers, i.e., A, B, C or D, the rate of evaporation will be the highest? Explain.
Answer: To determine the rate of evaporation, you need to consider factors such as surface area, temperature, and airflow. The container with the largest surface area exposed to air, highest temperature, or greatest airflow will have the highest rate of evaporation. For instance, if container A has a wide mouth and is placed in a warm, breezy environment, it will have a higher evaporation rate compared to others.
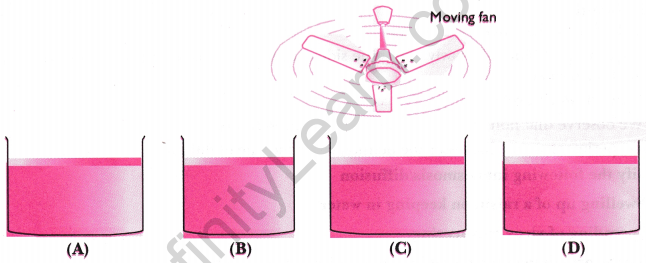
Question 14: Why do wet clothes dry quickly in the sun than in the shade?
Answer: Wet clothes dry faster in the sun because the temperature in sunny areas is higher than in the shade. Higher temperatures increase the rate of evaporation, causing the water in the clothes to evaporate more quickly.
Question 15: Why do trees acquire more leaves during summer?
Answer: During summer, the temperature is generally very high. To keep cool, trees must transpire more, which is the evaporation of water from their leaves. Increased transpiration requires more leaves, so trees acquire more leaves during summer to facilitate this process.
Question 16: Why do we feel comfortable under a fan when we are perspiring?
Answer: When we perspire, the sweat on our skin evaporates, a process that cools us down. The air from the fan increases the rate of evaporation, making us feel more comfortable and cool.
Question 17: Why do people sprinkle water on the roof after a hot sunny day?
Answer: Sprinkling water on the roof causes it to evaporate, absorbing a large amount of latent heat from the surface. This process cools the roof and makes the surroundings more comfortable.
Question 18: It is a hot summer day, Priyanshi and Ali are wearing cotton and nylon clothes respectively. Who would be more comfortable and why?
Answer: Priyanshi would be more comfortable because cotton absorbs sweat from the body and provides a larger surface area for evaporation, which cools the body. Nylon, on the other hand, does not absorb sweat as effectively, making it less comfortable in hot conditions.
- Chapter 1 Matter in Our Surroundings
- Chapter 2 Is Matter Around Us Pure
- Chapter 3 Atoms and Molecules
- Chapter 4 Structure of The Atom
- Chapter 5 The Fundamental Unit of Life
- Chapter 6 Tissues
- Chapter 7 Diversity in Living Organisms
- Chapter 8 Motion
- Chapter 9 Force and Laws of Motion
- Chapter 10 Gravitation
- Chapter 11 Work and Energy
- Chapter 12 Sound
- Chapter 13 Why Do We Fall ill
- Chapter 14 Natural Resources
- Chapter 15 Improvement in Food Resources
Matter in Our Surroundings Class 9 Extra Questions Short Answer Questions-II
Question 1: Substance ‘A’ has high compressibility and can be easily liquefied. It can take up the shape of any container. Predict the nature of the substance and list four properties of this state of matter.
Answer: ‘A’ is a gas.
Properties of gases:
- No Fixed Shape or Volume: Gases do not have a definite shape or volume and take the shape of their container.
- Large Interparticle Space: Gases have large spaces between particles compared to solids and liquids.
- Weak Intermolecular Forces: The forces of attraction between gas molecules are very weak.
- High Compressibility: Gases can be easily compressed due to the large gaps between particles.
Question 2: Suggest an activity to show that the rate of diffusion of liquids decreases with an increase in the density of the liquid.
Answer: Activity:
- Take two beakers filled with water.
- Slowly add a drop of blue ink along the side of the first beaker.
- Add a drop of honey in the same manner to the second beaker.
- Leave both beakers undisturbed and observe.
Observation:
- The blue ink spreads quickly throughout the water.
- The honey spreads much more slowly compared to the ink.
Conclusion: This experiment shows that the rate of diffusion is faster in liquids with lower density (ink) and slower in liquids with higher density (honey).
Question 3: Classify the following into osmosis or diffusion:
- Swelling up of a raisin on keeping in water.
- Spreading of virus on sneezing.
- Earthworm dying on coming in contact with common salt.
- Shrinking of grapes kept in thick sugar syrup.
- Preserving pickles in salt.
- Spreading of smell of cake being baked throughout the house.
- Aquatic animals using oxygen dissolved in water during respiration.
Answer:
- Osmosis
- Diffusion
- Osmosis
- Osmosis
- Osmosis
- Diffusion
- Diffusion
Question 4: Explain what happens to the molecular motion and energy of 1 kg of water at 273 K when it is changed into ice at the same temperature. How is the latent heat of fusion related to the energy exchange that takes place during this change of state?
Answer:
- Molecular Motion: As water changes to ice, the molecular motion decreases. The molecules move less freely and arrange into a more ordered, rigid structure.
- Energy Exchange: During this process, the water releases energy in the form of latent heat of fusion, which is the energy required to change the state without changing the temperature.
- Latent Heat of Fusion: The latent heat of fusion is the energy released when 1 kg of water at 273 K turns into ice at the same temperature. This energy is used to overcome the forces holding the water molecules in a liquid state and to arrange them into a solid structure.
Question 5. Design an experiment to show that ammonium chloride undergoes sublimation.
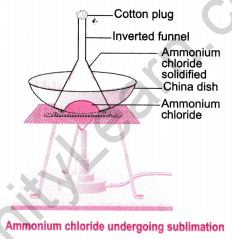
Answer:
- Take crystals of ammonium chloride in a china dish.
- Place the china dish on a tripod stand with a wire gauze.
- Cover the china dish with an inverted funnel and insert a cotton plug in the stem of the funnel.
- Heat the china dish on a low flame.
- Observe the inside of the funnel where white deposits of ammonium chloride appear, indicating it has sublimed directly from a solid to a gas and then solidified upon cooling.
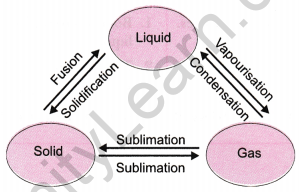
Question 6: Explain interconversion of three states of matter with the help of a flow chart. Name the process of each interconversion.
Answer: The interconversion of the three states of matter can be illustrated as follows:
- Solid to Liquid: Melting
- Liquid to Gas: Vaporization
- Gas to Liquid: Condensation
- Liquid to Solid: Freezing
- Solid to Gas: Sublimation
- Gas to Solid: Deposition
Question 7: A student heats a beaker containing ice and water. He measures the temperature of the content of the beaker as a function of time. Which of the following graphs correctly represents the result? Justify your choice.
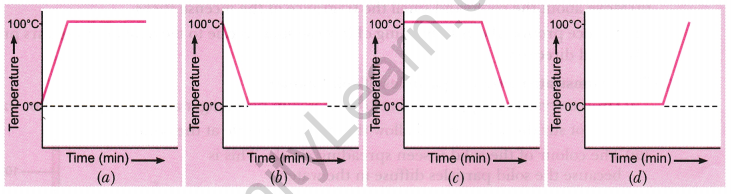
Answer: Since ice and water are in equilibrium, the temperature remains at zero degrees Celsius. Upon heating, the energy supplied is used in melting the ice, and the temperature remains constant until all the ice has melted due to the latent heat of fusion. After all the ice melts, the temperature of the water increases. Therefore, the correct graph is option (d), which shows a constant temperature during the phase change, followed by a rise.
Question 8: Explain how the rate of evaporation of a liquid is affected with:
- Increase in temperature of the liquid.
- Decrease in exposed surface area.
- Increase in moisture in the surrounding air.
- Increase in wind speed.
Answer:
- Increase in temperature of the liquid: The rate of evaporation increases because higher temperatures provide more energy to the liquid molecules, enabling them to escape into the gas phase.
- Decrease in exposed surface area: The rate of evaporation decreases as fewer molecules are exposed to the air, reducing the number of molecules that can evaporate.
- Increase in moisture in the surrounding air: The rate of evaporation decreases because the air is already saturated with water vapor, making it harder for more molecules to evaporate.
- Increase in wind speed: The rate of evaporation increases because wind removes the evaporated molecules from the surface, allowing more molecules to escape from the liquid.
Question 9: You want to wear your favourite shirt to a party, but the problem is that it is still wet after a wash. What steps would you take to dry it faster?
Answer: To dry the shirt faster, you can:
- Increase the surface area: Spread the shirt out to maximize the area exposed to air.
- Increase the temperature: Place the shirt under direct sunlight to increase the temperature, which enhances evaporation.
- Increase wind speed: Hang the shirt under a fan to increase the air flow around it, speeding up the evaporation process.
Question 10: How does evaporation differ from boiling?
Answer:
| Evaporation | Boiling |
| 1. Evaporation takes place at all temperatures. | 1. Boiling takes place only at the boiling point of the liquid. |
| 2. Temperature changes during evaporation. | 2. The temperature does not change during boiling. |
| 3. It is a very slow process. | 3. It is a fast process. |
| 4. Evaporation takes place only at the surface of the liquid. | 4. Boiling takes place in the entire body of the liquid. |
Matter in Our Surroundings Class 9 Extra Questions Long Answer Questions
Question 1: Describe the continuous motion of particles of matter with the help of an activity.
Answer: (a) Motion of particles in air:
- Place a few lighted incense sticks in a corner of a room.
- Move around the room and observe the fragrance spreading.
- The fragrance spreads due to the rapid movement of vapors in all directions, demonstrating the motion of particles in air.
(b) Motion of particles of solid matter:
- Drop a crystal of copper sulphate or potassium permanganate into a glass of hot water.
- Do not stir the solution and allow the crystals to settle.
- The color spreads slowly, showing that solid particles diffuse in water due to the motion of particles.
Question 2: Describe an activity to determine the boiling point of water and melting point of ice.
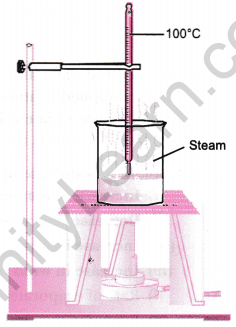
Answer: (a) Boiling point of water:
- Take water in a beaker and insert a thermometer with the help of a clamp.
- Heat the beaker on a tripod stand using a kerosene burner.
- Observe the temperature when a steady stream of bubbles forms, indicating the boiling point of water.
(b) Melting point of ice:
- Place crushed ice in a beaker and insert a thermometer, ensuring the bulb is inside the ice.
- Record the temperature at intervals until the ice starts melting.
- Continue recording the temperature until it remains constant, which is the melting point of ice.
Question 3: While heating ice in a beaker with a thermometer suspended in it, a student recorded the following observations:

Based on these, answer the following: (a) State the change observed between 2-3 min and name the process. (b) Between 30-35 min, the temperature remains constant. Explain why and name the heat involved.
Answer: (a) Between 2-3 min, ice changes into water, a process called fusion. (b) Between 30-35 min, the temperature remains constant as the heat supplied is used to overcome the intermolecular forces to change the liquid into vapor. This heat is known as latent heat of vaporization.
Question 4: Discuss the factors affecting the rate of evaporation. Given the latent heat of evaporation for two liquids, A and B, is 100 J/kg and 150 J/kg respectively, which one produces more cooling effect and why?
Answer: Factors affecting evaporation:
- Surface area: Larger surface area increases the rate of evaporation.
- Temperature: Higher temperature increases the rate of evaporation.
- Humidity: Higher humidity decreases the rate of evaporation.
- Wind speed: Higher wind speed increases the rate of evaporation.
- Nature of liquid: Volatile liquids evaporate faster than less volatile ones.
Liquid B produces more cooling because it absorbs more heat from the surroundings for evaporation due to its higher latent heat.
Question 5:
Comment on the following statements:
- Evaporation causes cooling.
- Rate of evaporation of an aqueous solution decreases with increase in humidity.
- Sponge though compressible is a solid.
- Ice is solid at 0°C, while water is liquid at room temperature.
- Sugar crystals dissolve faster in hot water than cold water.
Answer:
- Evaporation causes cooling because particles at the surface gain energy from the surroundings and convert to vapor, reducing the temperature.
- Rate of evaporation decreases with humidity because the air cannot hold more water vapor if it is already saturated, slowing down evaporation.
- Sponge is a solid despite being compressible due to its trapped air and flexible material. It retains its shape and volume when not compressed.
- Ice is solid at 0°C due to strong intermolecular forces giving it a definite shape and volume. Water is liquid at room temperature due to weaker forces, giving it a definite volume but no fixed shape.
- Sugar dissolves faster in hot water because the higher kinetic energy of hot water molecules allows them to break down sugar particles more quickly than cold water molecules.
Matter in Our Surroundings Class 9 Extra Questions Hots (Higher Order Thinking Skills)
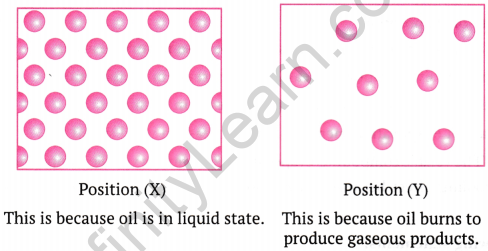
Question 1: The diagram below shows the burning of an oil lamp. Draw the arrangement of particles at positions ‘X’ and ‘Y’ when the lamp is burning.
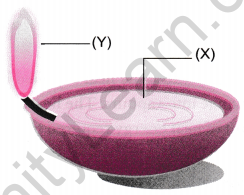
Draw the arrangement of particles of position ‘X’ and ‘Y’ when the lamp is burning.
Answer:
- At position ‘X’ (close to the flame), particles are in a gaseous state, moving rapidly and freely.
- At position ‘Y’ (in the oil), particles are in a liquid state, closely packed but still able to slide past each other.
Question 2: A small volume of water in a kettle can fill a kitchen with steam’. Explain why.
Answer: When water is heated, it converts from liquid to steam, a gaseous form. In this state, its particles move rapidly in all directions, filling the entire space available, such as the kitchen.
Question 3: A sample of water under study was found to boil at 102°C at normal temperature and pressure. Is the water pure? Will this water freeze at 0°C?
Answer: The water is not pure, as pure water boils at 100°C under normal conditions. The presence of impurities raises the boiling point and lowers the freezing point, so this water will freeze below 0°C.
Question 4: You are given the following substances with their melting and boiling points.
| Substance | Melting point (°C) | Boiling point (°C) |
| X | -219 | -183 |
| Y | 119 | 445 |
| Z | – 15 | 78 |
Question 5.
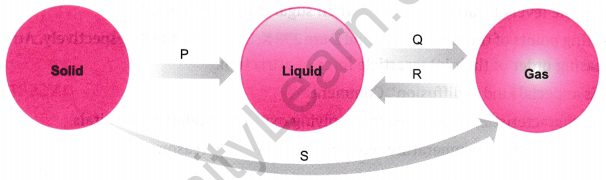
(a) Name the changes in terms of process P, Q, R, and S. (b) Which of the changes are exothermic and endothermic?
Answer: (a)
- P is fusion (melting).
- Q is boiling.
- R is condensation.
- S is sublimation.
(b)
- P, Q, and S are endothermic processes (absorbing heat).
- R is an exothermic process (releasing heat).
Question 6:
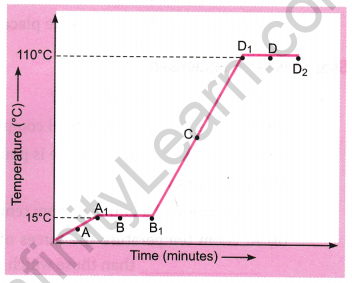
The temperature-time graph given alongside shows the heating curve for pure wax.
From the graph, answer the following:
(a) What is the physical state of the substance at points A, B, C, and D?
(b) What is the melting point of the substance?
(c) What is its boiling point?
(d) Which portions of the graph indicate that a change of state is taking place?
(e) Name the terms used for the heat absorbed during the change of states involved in the above process.
Answer: (a)
- A: Solid state.
- B: Both solid and liquid states.
- C: Both liquid and gaseous states.
- D: Liquid state.
(b) The melting point is 15°C.
(c) The boiling point is 110°C.
(d) A1B1 and D1D2 portions indicate a change of state.
(e) A1B1 represents the latent heat of fusion. D1D2 represents the latent heat of vaporization.
Question 7: Water as ice has a cooling effect, whereas water as steam may cause severe burns. Explain these observations.
Answer: Ice has low-energy water molecules, which absorb heat from the surroundings, providing a cooling effect. Steam, on the other hand, has high-energy molecules. When steam comes into contact with skin, it transfers a significant amount of energy as heat, potentially causing burns.
Benefit of Solving Matter in Our Surroundings Class 9 Extra Questions
- Enhanced Understanding: Solving extra questions deepens comprehension of matter, states of matter, and changes in its surroundings.
- Exam Preparation: Practice with extra questions prepares students for exams by covering essential topics thoroughly.
- Skill Development: Solving varied questions enhances analytical skills and boosts confidence in the subject.
- Concept Grasping: Extra questions aid in grasping concepts effectively, crucial for answering diverse questions in exams.
- Time Management: Practice with extra questions helps students assess their exam time management and revise the entire syllabus efficiently.
FAQs on Class 9 Science Chapter 1 Extra Questions and Answers
What forms our surroundings?
Everything around us, including air, water, plants, animals, and objects, is made up of matter and forms our surroundings.
How many types of matter are in our surrounding?
Matter in our surroundings exists in three states: solid, liquid, and gas. These three states have different properties and can be interconverted by changing temperature and pressure.
What is the theory of matter in our surroundings?
According to the theory of matter, everything around us is composed of tiny particles called atoms and molecules. These particles are in constant motion and have forces of attraction between them.
What are changes in our surroundings called?
Changes in our surroundings can be either physical or chemical. Physical changes do not involve the formation of new substances, while chemical changes result in the formation of new substances with different properties.
What are 5 examples of matter in our surroundings?
Five examples of matter in our surroundings are air, water, sand, wood, and plastic.
What is matter in a surrounding class 9?
Matter in our surroundings is the first chapter of Class 9 Science. It covers the fundamental concepts of matter, its states, and the changes it undergoes in our surroundings.