Table of Contents
Table of Contents
- Oxidation of a Substance
- Reduction of Substance
- Oxidation-Reduction Reaction
- Summary
- What’s Next?
In the previous segment, we learnt about balancing the chemical equations. In this segment, we will learn the concept of oxidation and reduction reactions.
What is Oxidation of a substance?
Oxidation of a substance occurs when it Gains Oxygen during a reaction and the substance is said to be oxidised.
What is Reduction of a substance?
Reduction of a substance occurs when it Loses Oxygen during a chemical reaction and the substance is called reduced.
What is an Oxidation-reduction reaction?
Redox reactions are chemical reactions where one reactant is gaining oxygen and gets oxidised, while the other gets reduced by losing oxygen. Such reactions are also called oxidation-reduction reactions.
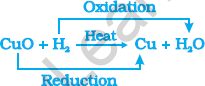
Summary
Redox reaction
| Oxidation | The process through which a substance gets oxidised |
| Reduction | The process through which a substance gets reduced |
| Oxidation- Reduction reaction | Chemical reaction where one reactant gets oxidised, while the other gets reduced |






