Table of Contents
- Metallic and Non-metallic Characters
- Summary
- Did You Know?
- What’s Next?
In the previous segment of the chapter ‘Periodic Classification of Elements’, we learnt about the
atomic size. In this segment, let us get introduced to the metallic and non-metallic characters.
What are Metallic and non-metallic characters in the periodic table?
- The modern periodic table has metals placed on the left, non-metals on the right, and the metalloids occupy the nearly central region of the table.
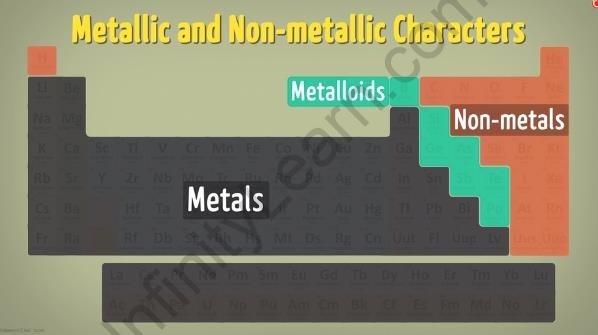
Metallic and non-metallic characters
- Metals tend to lose electrons because it is easier to lose electrons when they are very few in number rather than accepting more number of electrons.
- The atomic size increases down the group. The force of attraction from the nucleus for the valence electrons decreases. Thus, the elements show a tendency to lose electrons easily. So, the metallic character increases as we go down the group.
For example, fluorine and chlorine show better non-metallic properties but the metallic characters keep increasing down the group.

