Table of Contents
The number of connections an element can make changes as we move from the left to the right within a row on the periodic table. It goes up and then down, but when you look at elements in the same column, it stays the same.
In the previous segment of the chapter ‘Periodic Classification of Elements’, we studied placing the elements in the periodic table. In this segment, let us get introduced to valency.
What are the Trends in the modern periodic table?
Trends refer to the properties in the periodic table. The trends important for studying the elements are:
- Valency
- Atomic size
- Metallic and Non-metallic characters
What is Valency?
- The capacity of the atom to give or take electrons during bond formation is called
Valency.
- The atom, having one electron in its outermost shell, wants to donate or lose that electron. The atom, having 7 electrons in the outermost orbit, tends to accept or gain an electron from outside. The valency of both will be one because the first atom can donate one electron and the second one can accept 1 electron.
1 electron → Donate 1 electron 7 electrons → Accept 1 electron
- In any particular group, elements have the same valency.
- As we move down the group, the valency of elements remains the same because the number of valence electrons is the same throughout the group.
For example, halogens belonging to the 17th column have the same valency. From fluorine to iodine, there are the same number of valence electrons in their outermost orbital,
seven. All these elements show the valency one because they accept one electron from the other elements.
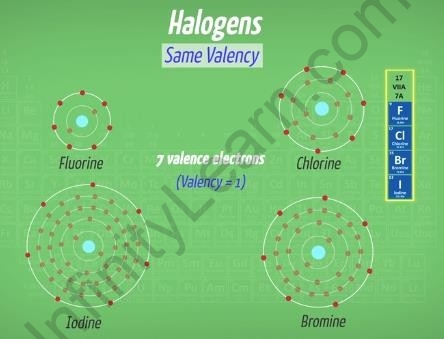
Halogens
- There is a difference in the valency of elements belonging to the same period.
- Metallic character of elements decreases from left to right in the period.
- The energy required to remove electrons from the atom is called Ionisation energy.
- Metals tend to give away electrons because they have low ionisation energy.
- Metals have freely available electrons that can be easily lost to attain a stable electronic configuration. This tendency is shown by elements like sodium or magnesium which are placed towards the left in the table.
- Elements towards the left have lesser valence electrons so they give away electrons easily.
Modern Periodic Table – Valency FAQs
Why does valency increase in a period?
Valency increases in a period because as you move across the periodic table, the number of electrons in the outermost shell stays the same, but the number of protons in the nucleus increases, making it easier for atoms to lose or gain electrons.
What is valency explain the trends in valency in a period and in a group?
Valency is how atoms combine with other atoms. In a period (horizontal row), valency tends to increase from left to right, while in a group (vertical column), valency often stays the same. Atomic size generally decreases from left to right across a period.
What is the trend in atomic size in the modern periodic table?
In the modern periodic table, atomic size tends to decrease from left to right across a period, as the number of protons in the nucleus increases, pulling the electrons closer.
What is the periodic trend of valency and atomic size?
The periodic trend for valency often increases from left to right in a period, while atomic size tends to decrease. In a group, valency may remain the same, and atomic size generally increases as you go down the group.









