Table of Contents
Introduction: Electrochemical series – 2
A set of standard electrode potentials has been constructed by measuring the potentials of various electrodes vs the standard hydrogen electrode (SHE). The electrochemical, electromotive, or activity series of the elements is formed when the electrodes (metals and non metals) in contact with their ions are organized based on the values of their standard reduction potentials or standard oxidation potentials.
Electrochemical series
An electrochemical series, also known as an activity series, is a list that describes the order in which elements are arranged in order of increasing electrode potential values. The series was created by comparing the potential of different electrodes to that of a conventional hydrogen electrode (SHE). The electrodes (metals and non-metals) in contact with their ions in an electrochemical series are organized according to the values of their standard reduction or oxidation potentials. When the half cell is connected to the standard hydrogen electrode under standard conditions, the voltage is measured to produce the standard electrode potential.
Elements that are both electropositive and electronegative
- Electropositive elements (other than hydrogen) are those that have a higher tendency to lose electrons to their solution. In any event, we can figure out the order in which metals will replace one another from their salts by looking at the electrochemical series. As a result, electropositive metals are commonly used to replace hydrogen in acids.
- Electronegative elements, on the other hand, are those that gain electrons. They are commonly found after the element hydrogen in the periodic table.
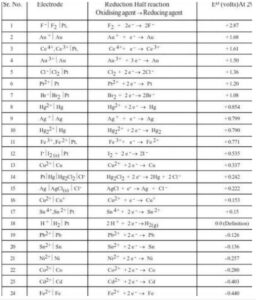
Chart of electrochemical series
The Electrochemical series PDF graphic is a quick method to see how similar and dissimilar metals are related. For roofers, it’s important to understand what material compatibility difficulties exist. This graph depicts both common and rare roofing metals. In the most basic terms, metals that are farther apart on this scale have a higher proclivity for corrosion than metals that are closer together. (i.e. Zinc and Copper are on opposite sides of the scale.) This means that a copper pipe would never drain water onto a zinc-coated roof).
Application of electrochemical series
- Strength of oxidizing and reducing : The electrochemical series aids in the identification of a good oxidising or reducing agent. All of the substances at the top of the electrochemical series are good oxidising agents, meaning they have a positive standard reduction potential, whereas those at the bottom of the electrochemical series are good reducing agents, meaning they have a negative standard reduction potential. F2, for example, is a strong oxidising agent with a standard reduction potential of +2.87 volts, while Li+ is a strong reducing agent with a standard reduction potential of -3.05 volts.
- Electrochemical Cell Standard Emf (E0) Calculation : The total of the standard reduction potentials of the two half cells is the cell’s standard emf: Half-cells for reduction and oxidation Eocell is made up of two words: Eored and Eoox. The standard oxidation potential is always represented in terms of reduction potential, as is customary. As a result, the standard oxidation potential (Eoox) equals – the standard reduction potential (Eored).
- Predicting redox reaction feasibility: If the free energy change (G) is negative, any redox reaction will occur spontaneously. The number of electrons involved is n, the Faraday constant is F, and the cell emf is Eo. If Eo is positive, Go can be negative. The cell response is spontaneous when Eo is positive, and it acts as a source of electrical energy. If the result is negative, the spontaneous reaction will not be possible. While determining the stability of a metal salt solution when stored in another metal container, the consequent value of Eo for redox reaction is crucial.
- Predicting the electrolysis product: If there are two or more types of positive and negative ions in solution, certain metal ions are discharged or freed at the electrodes in preference to others during electrolysis. In such a competition, the ion with the higher standard reduction potential (stronger oxidizing agent) is discharged first at the cathode. When an aqueous solution of NaCl containing Na+, Cl-, H+, and OH- ions is electrolyzed, the H+ ion is preferentially deposited at the cathode (reduction) rather than the Na+ ion, since hydrogen’s reduction potential (0.00 volt) is larger than sodium’s reduction potential (0.00 volt) (-2.71 volt). The anion with the lowest reduction potential will be present at the anode where oxidation occurs. As a result, OH-, which has a standard reduction potential of 0.40 volt, will be oxidised first, followed by Cl-, which has a standard reduction potential of 1.36 volt.
Important points in electrochemical series:
- The following are some key elements to remember from this lecture.
- The electrochemical series considers an element’s reduction potential in terms of the hydrogen scale, where Eo = zero. The standard reduction potential of an element is defined as a measure of an element’s likelihood to undergo reduction, according to the definition.
- The larger an element’s reduction potential, the easier it is to reduce it. Elements with a low reduction potential, on the other hand, will oxidise much more quickly and easily.
- Elements that readily give up electrons, on the other hand, have a negative or lower reduction potential. Positive) or higher reduction potential elements are those that do not readily give away electrons but readily receive them.
- In the electrochemical series, stronger reducing agents with negative standard reduction potential are frequently found below hydrogen. Weaker reducing agents with a positive standard reduction potential, on the other hand, are located above the hydrogen in the series.
- The strength of the reducing agent grows as we progress down the group, whereas the strength of the oxidising agent diminishes.
- Similarly, as we progress through the series, the electro positive and activity of metals increases or intensifies. It lowers in the case of non-metals.
FAQs
What is the electrochemical series and how does it work?
Ans 1: The electrochemical series is listed in the table below. 'The Electrochemical Series': Standard Aqueous Electrode Potentials at 25°C The negative sign of the standard reduction potential implies that when an electrode is connected to SHE, it becomes an anode and oxidation happens.
In an electrochemical series (ECS), how are elements organized?
Elements have been grouped in electrochemical series (ECS) in order of increasing standard reduction potential commencing from the most negative to the most positive value w.r.t. SHE (E° she = 0.00 V).
How can I remember the electrochemical series more quickly?
There are numerous songs and stories that can assist you in memorizing the electrochemical series more quickly. Here are a few illustrations. Perhaps one of them will inspire you to write your own song, narrative, or illustration to help you recall the series more quickly.