Table of Contents
What is Blue Vitriol?
Blue vitriol, commonly known as copper(II) sulfate pentahydrate, is a widely used inorganic compound characterized by its bright blue crystalline appearance. It is also referred to as bluestone or Roman vitriol.
Blue Vitriol Chemical Name
The chemical name of blue vitriol is copper(II) sulfate pentahydrate. This name reflects its composition, which includes copper, sulfur, and water molecules.
Blue Vitriol Chemical Formula
The chemical formula of blue vitriol is CuSO4·5H2O. This indicates that each molecule of copper sulfate is associated with five molecules of water.
Also Read: Science Topics
Blue Vitriol Molecular Formula
The molecular formula for blue vitriol, CuSO4·5H2O, highlights its hydrated nature, crucial for its various physical and chemical properties.
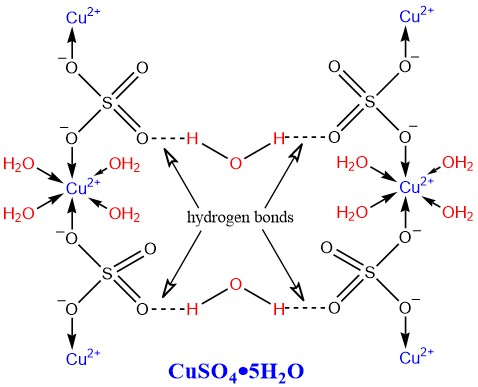
Blue Vitriol Structure
In the structure of blue vitriol, copper is coordinated to four water molecules and one sulfate ion. The fifth water molecule is hydrogen-bonded to the sulfate ion.
Blue Vitriol Uses
Blue vitriol has several uses, including:
- As a fungicide and herbicide in agriculture.
- In the electroplating and battery industries.
- For educational purposes in chemistry laboratories.
Is Blue Vitriol Soluble in Water
Blue vitriol is highly soluble in water, which is why it is often used in aqueous solutions for various industrial applications.
Conclusion
Blue vitriol, with its chemical formula CuSO4·5H2O, plays a significant role in various industrial and educational fields due to its distinct properties and versatile applications. Understanding its structure and uses helps in appreciating its importance in both scientific and practical contexts.
| Science Topics | |
| Mercury Metal | Nitrogen |
| Magnesium | Calcium |
| Silver | Chlorine |
| Mercury Element | Helium |
| Oxygen | Gold |
Frequently Asked Questions (FAQs)
What is the crystal structure of blue vitriol?
The crystal structure of blue vitriol is triclinic. It features copper ions coordinated with water molecules and sulfate ions.
Why is CuSO4·5H2O called blue vitriol?
CuSO4·5H2O is called blue vitriol due to its characteristic bright blue color and historical use as a vitriolic substance.
What is the blue vitriol formula?
The chemical formula of blue vitriol is CuSO4·5H2O.
Which is called blue vitriol?
Copper(II) sulfate pentahydrate (CuSO4·5H2O) is called blue vitriol.
Is CuSO4 a blue vitriol?
CuSO4 without water is anhydrous copper sulfate, while CuSO4·5H2O is blue vitriol.
Write the chemical formula of blue vitriol.
The chemical formula of blue vitriol is CuSO4·5H2O.
Does blue vitriol contain water of crystallization?
Yes, blue vitriol contains five molecules of water of crystallization.
What happens when blue vitriol is heated?
When heated, blue vitriol loses its water of crystallization and turns into anhydrous copper sulfate, which is white.
What is blue vitriol and green vitriol?
Blue vitriol is copper(II) sulfate pentahydrate (CuSO4·5H2O), and green vitriol is iron(II) sulfate heptahydrate (FeSO4·7H2O).








