Table of Contents
The phenolphthalein indicator is a pH indicator solution that appears colorless in acidic and very alkaline conditions but turns fuchsia pink in normal alkaline conditions. This guide covers the colors seen at different pH levels, the chemical process behind the color change, how to create a phenolphthalein indicator, and its various uses. Here, we will explore the colors at different pH ranges, understand the chemistry of the color change, learn how to make a phenolphthalein indicator, and discover the uses of this chemical.
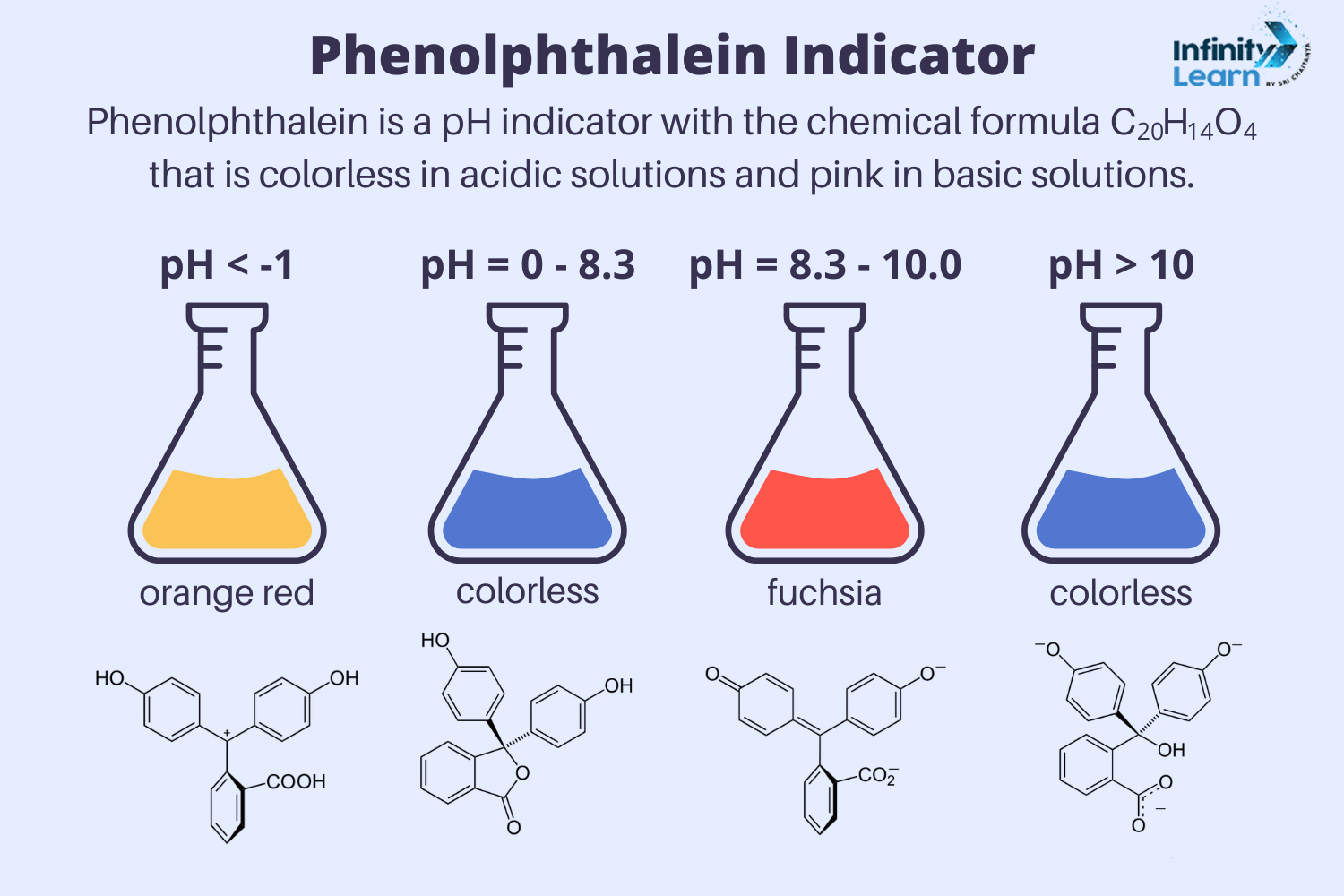
How Phenolphthalein Indicator Work?
The pH scale ranges from 0 to 14 and helps detect acids and bases. Acids fall between 0 and 6.9 on the pH scale, with lower numbers indicating stronger acidity. A pH of 7 is neutral, like the pH of water. Bases are found between 7.1 and 14, with higher numbers indicating stronger basicity. Chemists often use litmus paper to test the pH of a substance. Litmus paper turns red in contact with acids and blue when it touches bases.
The phenolphthalein indicator, unlike litmus paper, is naturally colorless. It changes color differently: it turns pink in an alkaline solution or base. In acids, the phenolphthalein indicator remains colorless but starts to turn pink at a pH of 8.2, becoming bright purple in strong bases. This unique property shows how the phenolphthalein indicator works effectively to identify the pH of a solution. Understanding how the phenolphthalein indicator works, especially the behavior of the phenolphthalein indicator in acid and base, is crucial for various chemical applications.
Also Check: Magnesium
How to Make Phenolphthalein Indicator Solution
You can buy pre-mixed phenolphthalein indicator solution, but it’s quite simple to make on your own. Phenolphthalein is somewhat soluble in water (400 mg/L), but it dissolves much better in ethanol or ether. Typically, you first dissolve the phenolphthalein powder in alcohol and then dilute it with water or use a 50% alcohol solution.
Here’s the recipe for a 1% phenolphthalein indicator solution:
- Weigh out 1.0 grams of phenolphthalein.
- Dissolve it in 100 milliliters of 50% ethanol in water. Alternatively, dissolve it in a mix of 50 milliliters of absolute ethanol and 50 milliliters of water.
For a 0.5% phenolphthalein indicator solution:
- Weigh out 0.5 grams of phenolphthalein.
- Dissolve it in 50 milliliters of ethanol.
- Add distilled water to reach a final volume of 100 milliliters.
The phenolphthalein indicator changes color based on the pH of the solution it’s in. In acidic solutions, phenolphthalein indicator remains colorless, while in basic solutions, it turns pink. Understanding how the phenolphthalein indicator works helps in various applications, such as titrations in chemistry. Knowing how phenolphthalein indicator behaves in acid and how phenolphthalein indicator works is crucial for accurate results in experiments.
Phenolphthalein Indicator Colour
Ionization causes the colour change in the Phenolphthalein indicator. This happens when a molecule gains or loses an electron, resulting in a positive or negative charge. An ionized molecule attracts opposite charges and repels similar ones, which changes its shape and affects how it interacts with light.
In a neutral solution, the Phenolphthalein indicator appears colourless because it allows all colours of light to pass through. However, in an alkaline solution, the Phenolphthalein indicator starts blocking blue light, causing the solution to turn pink. The stronger the alkaline solution, the more the Phenolphthalein indicator molecules change, resulting in a darker pink colour. Thus, the phenolphthalein indicator colour varies depending on the alkalinity of the solution.
FAQs on Phenolphthalein Indicator
What Does the Phenolphthalein Indicator Indicate?
Phenolphthalein indicator (C20H14O4) is a widely used acid-base indicator from the phthalein family. It helps determine the pH of a solution. The phenolphthalein indicator is colorless below a pH of 8.5 but turns pink to deep red above a pH of 9.0.
What Turns the Phenolphthalein Indicator Pink?
The phenolphthalein indicator turns pink in basic solutions. When the solution is acidic, the indicator remains colorless. Therefore, if the phenolphthalein indicator turns pink, it shows that the solution is basic with a pH higher than 7.
What is the Color Change of the Phenolphthalein Indicator?
Phenolphthalein is naturally colorless. Unlike litmus paper, it turns pink in alkaline (basic) solutions. In acidic solutions, it stays colorless but begins to turn pink at pH 8.2, becoming bright purple in strong bases.
Is the Phenolphthalein Indicator Liquid or Solid?
The phenolphthalein indicator exists as a colorless crystalline solid. In its solid form, it has a melting point of 262.5°C. In solutions, it remains colorless in acids and turns pink in basic solutions.
Can You Drink the Phenolphthalein Indicator?
Ingesting the phenolphthalein indicator can be harmful. Although used in laxatives, large doses can cause nausea, vomiting, and diarrhea. There are no reports of systemic toxicity from oral doses, but occasional allergic reactions can occur.
How to Prepare the Phenolphthalein Indicator?
To prepare the phenolphthalein indicator, dissolve 10.0 grams of phenolphthalein powder in 750 milliliters of ethanol. Then, dilute the solution to 1 liter with distilled water. Add 0.1 N sodium hydroxide (NaOH) dropwise until the solution reaches a faint pink endpoint. The color and pH range of the phenolphthalein indicator is colorless at pH 8.3 and turns red up to pH 10.0.






