Table of Contents
Introduction to Benzoyl Chloride
Benzoyl chloride, also known as benzene carbonyl chloride, is an important organic compound that belongs to the family of acyl chlorides. It is widely used in various chemical reactions and plays a significant role in the synthesis of pharmaceuticals, agrochemicals, and other organic compounds.
Benzoyl chloride is a colourless, flaming liquid with no scent. It is an acyl halide chemical that is mostly utilised to create peroxides. It contributes to the development of cancer. It is an acyl chloride that belongs to the benzene family. In terms of function, it is similar to benzoic acid.
Although it is beneficial in many other disciplines, including the creation of colours, fragrances, pharmaceuticals, and resins, it is most effective in the synthesis of peroxides.
This article will explore the structure, formula, preparation methods, properties, and various applications of benzoyl chloride.
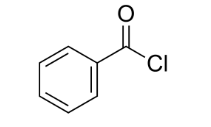
Benzoyl chloride formula and structure
The benzoyl chloride formula is C7H5ClO, and its structure consists of a benzene ring with a carbonyl group (C=O) attached to it. A chlorine atom (Cl) at the carbonyl carbon gives benzoyl chloride its characteristic properties and reactivity. The benzoyl chloride structure is shown as follows:
Preparation of Benzoyl Chloride
Benzoyl chloride is prepared from benzoic acid (C6H5COOH) by treating it with thionyl chloride (SOCl2) or phosphorus pentachloride (PCl5). The reaction involves the replacement of the hydroxyl group (-OH) of benzoic acid with a chlorine atom, resulting in the formation of benzoyl chloride.
PhCOOH + SOCl2 → PhCOCl + SO2 + HCl
Benzoyl Chloride Molecular Weight and IUPAC Name
The benzoyl chloride molecular weight is calculated as 140.57 g/mol. According to the International Union of Pure and Applied Chemistry (IUPAC) nomenclature, benzoyl chloride IUPAC name is benzene-1-carbonyl chloride.
Reactions of Benzoyl Chloride
Benzoyl chloride is a highly reactive compound and undergoes various chemical reactions due to the presence of the acyl chloride group. Some of the important reactions include:
- Hydrolysis:
Benzoyl chloride reacts with water (H2O) to form benzoic acid and hydrochloric acid (HCl) as byproducts. The reaction explaining benzoyl chloride to benzoic acid conversion shown below:
C6H5COCl + H2O → C6H5COOH + HCl - Reduction:
Benzoyl chloride on reduction with H2/Pd-BaSO4 produces benzaldehyde.
C6H5 + H2 → C6H5CHO + HCl - Amide Formation:
Benzoyl chloride reacts with ammonia (NH3) or amines to form benzamide or N-substituted benzamides.
C6H5 + NH3 → C6H5CONH2 + HCl - Ester Formation:
Benzoyl chloride reacts with alcohols to form benzoyl esters.
C6H5 + R-OH → C6H5COOR’ + HCl
Benzoyl chloride uses
Benzoyl chloride finds widespread applications in the chemical industry:
- Pharmaceutical Industry: It is used to synthesize various pharmaceutical compounds, including analgesics and anti-inflammatory drugs.
- Agrochemicals: Benzoyl chloride is used to produce pesticides and herbicides.
- Perfumery: It is used to synthesize aromatic compounds used in perfumery.
- Plastics: Benzoyl chloride is employed in manufacturing polymers and plastics.
Conclusion
Benzoyl chloride is a versatile and essential chemical compound with various applications in different industries. Its reactivity and unique properties make it an essential building block in the synthesis of numerous organic compounds.
Understanding the structure, formula, preparation methods, properties, and uses of benzoyl chloride provides valuable insights into its significance in organic chemistry.
Frequently Asked Questions on Benzoyl Chloride
What is the chemical formula for benzoyl chloride?
Benzoyl chloride, commonly known as benzene carbonyl chloride, is a C7H5ClO organochlorine chemical. It is a white, fuming liquid with an unpleasant stench that is made up of a benzene ring (C6H6) and an acyl chloride (C(=O)Cl) substituent.
What are the benefits of benzoyl chloride?
It is used in medicine and the production of other compounds. Benzoyl chloride is an acyl chloride formed by replacing a hydrogen atom in benzene with an acyl chloride group. It serves as a critical chemical step in the production of various compounds, dyes, fragrances, herbicides, and medicines.
What properties does benzoyl chloride have?
Benzoyl chloride is another name for benzene carbonyl chloride. C7H5ClO is the chemical formula for benzoyl chloride. When exposed to wet air, it is a white liquid with a boiling point of 198° and a pungent fragrance. It has a strong scent, and its vapours cause excessive watering of the eyes and nose.
How is benzyl chloride made?
Benzyl chloride is manufactured industrially through the gas-phase photochemical reaction of toluene with chlorine: C6H5CH3 + Cl3→C6H6CH2Cl + HCl Each year, around 100,000 tonnes are manufactured in this manner. The reaction proceeds via the free radical process, which involves the intermediation of free chlorine atoms.








