Table of Contents
Introduction to Bleaching Powder
Bleaching powder, also known as calcium hypochlorite, is a widely used chemical compound with diverse applications. It is a powerful oxidizing agent commonly employed as a bleaching and disinfecting agent.
Its effectiveness as a bleaching, disinfecting, and water-purifying agent makes it indispensable in modern-day applications. In this article, we will explore the formula, preparation methods, various uses, and properties of bleaching powder.
Chemical Formula of Bleaching Powder
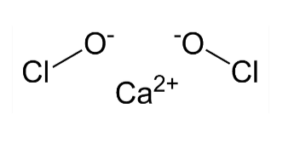
The chemical formula of bleaching powder is Ca(ClO)2. It is composed of calcium cations (Ca2+) and hypochlorite anions (ClO-). This formula indicates that each molecule of bleaching powder contains one calcium atom, two chlorine atoms, and two oxygen atoms.
Chemical Name of Bleaching Powder
The chemical name of bleaching powder is calcium hypochlorite. It is called so because it contains calcium (Ca2+) and hypochlorite (ClO-) ions.
Bleaching Powder Price
The bleaching powder price may vary depending on factors such as the purity of the product, the quantity purchased, and market conditions. It is available in different grades for various industrial and domestic purposes.
Preparation of Bleaching Powder
Bleaching powder is prepared by passing chlorine gas over dry slaked lime (calcium hydroxide). The reaction proceeds as follows:
Ca(OH)2 + Cl2 → Ca(ClO)2 + H2O
The resulting product, calcium hypochlorite, is the bleaching powder.
Properties of Bleaching Powder
- Odor: Bleaching powder has a strong, pungent odor due to the release of chlorine gas upon exposure to air.
- Appearance: It is a white to pale yellow powder or granules with a chlorine-like smell.
- Solubility: Bleaching powder is sparingly soluble in water, and its solubility decreases with an increase in temperature.
- Stability: It is relatively stable when stored in a dry and cool place. However, it should be protected from direct sunlight, as it can decompose into calcium chloride and chlorine gas.
- Oxidizing Property: Bleaching powder is a powerful oxidizing agent and releases oxygen when it comes into contact with reducing substances.
Bleaching Powder in Hindi
In Hindi, bleaching powder is known as “सफेदी” (Safedi), which directly translates to “whitener.” This name is derived from its prominent use as a whitening and bleaching agent.
Bleaching Powder Uses
- Bleaching Agent: One of the primary uses of bleaching powder is as a powerful bleaching agent in the textile and paper industries. It is also used to whiten fabrics, pulp, and paper.
- Water Purification: Bleaching powder is widely used to disinfect and purify water. It effectively kills harmful microorganisms, including bacteria and viruses, making water safe for consumption.
- Household Cleaning: In households, bleaching powder is utilized as a cleaning agent to remove stains and disinfect surfaces.
- Sanitizing Agent: It is employed in the sanitation of swimming pools and other water bodies to eliminate algae and harmful pathogens.
- Chemical Industries: Bleaching powder serves as an essential chemical in various industrial processes, such as the production of chloroform, chlorinated rubber, and other organic compounds.
Frequently Asked Questions on Bleaching Powder
What is Bleaching Powder?
Bleaching powder is a chemical compound that is widely used for its bleaching, disinfecting, and sanitizing properties. It is an essential product in various industries and plays a crucial role in water purification, textile processing, and cleaning processes.
What are the chemical and physical features of bleaching powder?
It is produced at 40 oC by the action of chlorine on dry slaked lime, Ca(OH)2. This is the Odling point of view about its formation. Clifford proposes another theory in which bleaching powder is a blend of calcium hypochlorite and basic calcium chloride.
What colour is bleaching powder?
Bleaching powder has a yellowish white colour. Bleaching powder is a white solid substance, but the colour of the powder that is used commercially is yellowish white rather than pure white.
What are the different kinds of bleaching powder?
When determining which bleach to use on your laundry, there are just two basic types: chlorine and oxygen bleach. There are, however, natural things that have bleaching activity and can be used as bleaching agents.
What causes the milky appearance of bleaching powder?
Bleaching powder dissolves in cold water and produces a milky solution due to the presence of lime, which appears milky in water.








