Table of Contents
Introduction to Decantation
We’ve all witnessed our mothers in the kitchen separating stones and husk from rice and pulse grains. Handpicking easily removes apparent contaminants such as stones and husks. But what about minor pollutants like mud and dust?
Water is poured into the vessel, and the top layer of unclean water is eliminated. Decantation is the process of separating solid contaminants from liquid solutions.
Read the article for more detail, which covers all of the foundations of this decantation process in the liquid form of matter. It goes into the definition, technique, and applications, among other things.
Definition of Decantation
Decantation meaning in English is the separation of both a liquid and solid mixture or the separation of immiscible liquid mixtures. Decantation separates water containing muck, oil and water, fat or grease on top of the soup, for example. This procedure separates the top liquid layer from the layer beneath it.
A decanter is a piece of glassware used to accomplish decantation. It comes in a variety of styles. The separation of vinegar and oil is another example of decantation. Decantation images explain this process clearly.
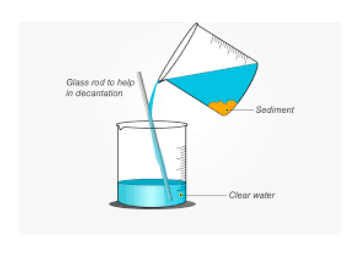
Decantation process
There are two types of decantation process:
Solid-Liquid Separation
In this procedure, the solid is initially allowed to settle at the bottom by gravity. The liquid is subsequently transferred to another vessel. A centrifuge is employed if the natural settling process is too slow.
The centrifuge can speed up the separation of solid impurities by applying more force to them. This operation makes use of loading. Alum is used to make suspended contaminants heavier during loading. Impurities settle down faster as they become heavier, resulting in a clear liquid.
Separation of Immiscible Liquids
This procedure is simple since it separates two immiscible liquids with different densities. When two immiscible liquids mix, two layers form. The lighter liquid rises to the surface of the heavier one.
As a result, the top layer (lighter liquid) is separated by emptying it into a separate vessel. Precision is required for this process because it is incredibly difficult to pour out the entire top layer without also leaking the bottom layer. Oil and water are separated using this procedure.
Decanting diagram
The explanatory decanting diagram is shown below:
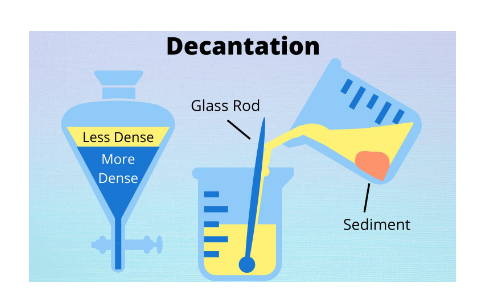
Loading process
The loading process is the separation of a combination of liquids and liquids containing microscopic particles by adding a chemical that adheres to the impurities and makes them heavy.
For example, if we take a little muddy sample and add some alum to it, we will notice that after some time, a layer of mud settles at the bottom of the jar, and the water will become clear. The alum bonded to the little bud particles, making them heavier and causing the mud to settle at the bottom. This method is used in water filtration tanks to filter water. The loading process is explained below by the image:
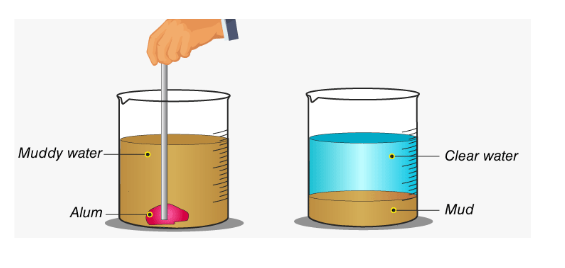
Decantation examples in everyday life
There are various Decantation examples in everyday life as mentioned below:
- Cheese/ cream and Milk
- Blood and Plasma
- Precipitate separation from Supernet
- Dirt and Water
Also Check For:
Frequently Asked Questions on Decantation
What is decantation?
Decantation is the process of separating liquids from solids and other liquids that are immiscible by eliminating the liquid layer on top from the solid or liquid layer beneath.
What are the applications of decantation?
Decantation is an effective way to separate different densities of immiscible liquids. When a mixture of oil and water is present in a beaker, for example, a distinct layer between the two liquids forms after some time, with the oil layer floating on top of the water layer.
What are the 10 examples of decantation?
Let's look at some real-world decantation examples:
Bottles of wine
Glycerin extraction from biodiesel
Mercury decontamination
Milk and cream
Sugar Beet Production
Nanotechnology
Fractionation of blood
Cooking
Manufacturing of Vinegar
Is salt and water an example of decantation?
No, because salt is soluble in water, sedimentation and decantation cannot be used to separate a mixture. However, sedimentation and decantation are capable of being used to separate a mixture of solids and liquids.
What is the procedure of beaker decantation?
Method/Procedure for Beaker Decantation
Choose the size of the cut.
Select the mineral's specific gravity.
Determine the viscosity of the fluid by measuring its temperature.
Determine the fluid's specific gravity.








