Table of Contents
Introduction to Osmosis and Diffusion
In the fascinating world of chemistry, two processes that often capture our attention are osmosis and diffusion. These two phenomena are essential for understanding the movement of molecules and particles within various substances.
In this article, we will embark on a journey to explore the difference between osmosis and diffusion class 12, unraveling their definitions, mechanisms, and real-life implications
Difference between osmosis and diffusion definition
Osmosis and diffusion are natural processes that govern the movement of particles, particularly molecules and ions, across different mediums.
There are various difference between osmosis and simple diffusion. Before diving into their distinctions, let’s gain a better understanding of the difference between osmosis and diffusion class 12.
Osmosis
Osmosis is a type of diffusion in which water is transferred from a high-concentration zone to a low-concentration zone over a semi-permeable membrane. Semi-permeable membranes include cell membranes.
Small molecules such as oxygen, water, carbon dioxide, and glucose can pass through cell membranes, but bigger molecules such as sucrose, proteins, and starch cannot.
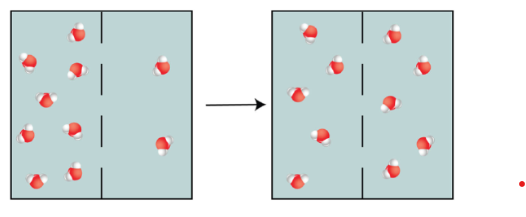
For example: if a semi-permeable membrane had more water molecules on one side than the other, water molecules would flow from the side with the higher concentration of water to the side with the lower concentration of water.
This process would be repeated until the concentration of water on both sides of the membrane was equal (dynamic equilibrium was reached).
Diffusion
Diffusion can be defined as the total movement of molecules from a higher concentration area towards a lower concentration area. The molecules in a gas, liquid, or solid are constantly moving due to their kinetic energy.
Molecules are continually moving and colliding with one another. Because of these collisions, the molecules move in random ways. However, additional molecules will be driven into the less concentrated area over time.
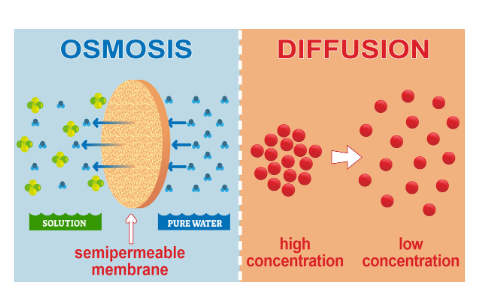
Difference between osmosis and diffusion with Diagram
The difference between osmosis and diffusion with diagram is as shown in the figure.
Difference between osmosis and diffusion in cell
Osmosis can only happen in a liquid medium, whereas diffusion can happen in any of the three (solid, liquid, or gas). Furthermore, whereas diffusion does not need the usage of a semi-permeable membrane, osmosis must. Water intake in plants is an example of osmosis. This is a major difference between osmosis and diffusion in cell.
Difference between Osmosis and Diffusion in tabular form
The difference between osmosis and diffusion in tabular form is as shown below:
| Osmosis | Diffusion |
| Limited to the liquid medium. | Occurs in liquid, gas, and even solids. |
| Requires a semipermeable membrane. | Does not require a semipermeable membrane. |
| Depends on the number of solute particles dissolved in the solvent. | Depends on the presence of other particles. |
| Requires water for the movement of particles. | Does not require water for the movement of particles. |
| Only the solvent molecules can diffuse. | Both the molecules of solute and solvent can diffuse. |
| The flow of particles occurs only in one direction. | The flow of particles occurs in all directions. |
| The process can either be stopped or reversed by applying additional pressure on the solution side. | This process can neither be stopped nor reversed. |
| Occurs only between similar types of solutions. | Occurs between similar and dissimilar types of solutions. |
| Involves the movement of only solvent molecules from one side to the other. | Involves the movement of all particles from one region to the other. |
| The concentration of the solvent does not become equal on both sides of the membrane. | The concentration of the diffusion substance equalizes to fill the available space. |
| Depends on solute potential. | Does not depend on solute potential, pressure potential, or water potential. |
| Only water or another solvent moves from a region of high concentration to a region of lower concentration. | Any type of substance moves from an area of highest energy or concentration to a region of lowest energy or concentration. |
| Not associated with the uptake of minerals and nutrients. | It helps in the uptake of minerals and nutrients. |
FAQs on Difference between Osmosis and Diffusion
What is the difference between osmosis and diffusion class 9 ?
Diffusion occurs when particles move from a higher concentration area to a lower concentration area until equilibrium is attained. Because osmosis uses a semipermeable barrier, just the solvent molecules are liberated to migrate to equalise concentration.
What is the simple definition of osmosis diffusion?
Osmosis is a sort of diffusion that involves the transfer of water from a high-concentration zone to a low-concentration region over a semi-permeable membrane.
How will you define diffusion?
Diffusion is the movement of molecules down a concentration gradient from a region of higher concentration to a region of lower concentration. This is why osmosis is called diffusion.
What are 5 differences between diffusion and osmosis?
Diffusion can happen in any material, liquid, solid, or gas. Only in a liquid medium can osmosis occur. The use of a semipermeable membrane is not required for diffusion. Osmosis necessitates the use of a semipermeable membrane.
What are the different types of diffusion and osmosis?
Diffusion is classified into two types: simple diffusion and facilitated diffusion. Regular osmosis and chemiosmosis are the two basic forms of osmosis.








