Table of Contents
Introduction to First order reaction
The concentration of a reactant influences the rate of various reactions. When we double the concentration, the rate at which it occurs also doubles. This is why such chemical reactions are referred to as first-order reactions.
In this article, we will go over first-order reactions in depth, including their definition, derivation, graph, and applications.
First order reaction formula
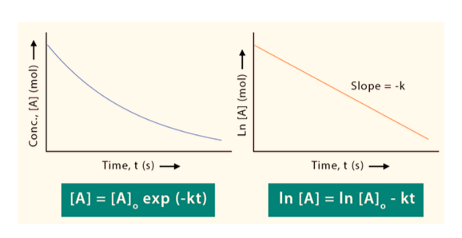
The equation ln[A] = -kt + ln[A]0 represents First order reaction formula which is comparable to that of a straight line (y = mx + c) with a slope of -k. This line can be represented graphically as follows. Thus, for a first-order reaction, the graph of ln[A] v/s t is a straight line with slope of -k.
First order reaction example
The following are some First order reaction examples:
Nitrogen Pentoxide (N2O5) decomposition: 2N2O5 → 2NO2 + 1/2 O2
Ammonium nitrate decomposition in aqueous solution: NH4NO2 → N2 + 2H2O
H2O2 decomposition in aqueous solution: H2O2 → H2O + 12 O2
Ethane hydrogenation: C2H4 + H2 → C2H6
Diazo derivative hydrolysis: C5H5N = NCl + H2O→ C6H5OH + N2 + HCl
First order reaction graph
The First order reaction graph is shown as below:
Rate constant for first order reaction and First order reaction units
The unit of rate constant for first order reaction is represented in reciprocal seconds (s1). The unit of rate constant for first order reaction can be calculated using the formula as follows:
k units = M(1-n).s-1 (where ‘n’ is the reaction order)
Because a first-order reaction has a reaction order of one, the equation is converted as follows:
k units = M(1-1).s-1 = s-1
Half life period of first order reaction
The Half life of first order reaction is the time it takes for the reactant concentration to fall to one-half of its initial value. The half life of first order reaction is independent of the reactant concentration. The half life of first order reaction is a constant that is connected to the reaction’s rate constant:
t1/2 = 0.693/k.
Integrated rate equation for first order reaction
Integrated rate equation for a first order reaction is ln[A]t = -kt + ln[A]0. Because this equation has the form y = mx + b, a straight line can be obtained by plotting the natural log of [A] as a function of time.
Also Check Related Topics:
Pseudo first order reaction
Pseudo first order reaction are not first-order but appear to be so because the reactant(s) are present at higher concentrations than other reactants.
In the rate law expression, the order of a chemical reaction can be stated as the product of the powers of the reactant concentrations.
Reactions are classified as first-order, pseudo-first-order, second-order, and so on based on the concentration of the reactant.
To calculate the reaction rate, accurate concentrations of each reactant are required.
If one or both of the essential reactants are expensive, the experiment may be expensive.
The Pseudo first order reaction, in which the 2nd order reaction is regarded as if it were a 1st order reaction, is used to avoid more complicated and costly experiments and calculations.
Explain pseudo first order reaction with suitable example
The reaction that is bimolecular yet has an order of one is known as a faux first order reaction. This happens whenever one of the reactants is in extremely high concentration. Pseudo first order reaction example is the acidic hydrolysis of an ester (ethyl acetate).
Frequently Asked Questions (FAQs) on First Order Reaction
What is a first order reaction, and what is an example of one?
The following are some examples of First order reactions: Nitrogen Pentoxide (N2O5) decomposition: 2N2O5 → 2NO2 + 1/2O. In aqueous solution, ammonium nitrate decomposes into NH4NO2 → N2 + 2H2O. In aqueous solution, H2O2 decomposes as H2O2 → H2O + 1/2 O.
What type of graph is a first order reaction?
A plot of the natural logarithm of a reactant's concentration versus time for a first-order reaction is a straight line having a slope of k. A plot of the inverse of a reactant's concentration versus time for a second-order reaction is a straight line having a slope of k.
What is the half life of second order reaction?
The half life of second order reaction is inversely proportional to the amount of the reactants, according to this equation.
Explain pseudo first order reaction with suitable example?
Pseudo first order reaction: A reaction that is bimolecular yet has an order of one is referred to as a pseudo first order reaction. This occurs when one of the reactants is in extremely high concentration. Pseudo first order reaction example is explained by acidic hydrolysis of an ester (ethyl acetate).








