Table of Contents
Introduction to Newman Projections
Stereochemistry covers all important aspects of stereochemistry, beginning with the history of stereochemistry and moving on to discuss molecular geometry in terms of bond distances and dihedral angles, as well as the biological importance of chirality and its effect on taste, odor, agrochemicals, and pharmaceuticals, before moving on to the basic principles, conformations, and configurations.
Definition of Newman projection
A Newman projection is a graphic that aids in the visualization of a molecule’s three-dimensional structure. This projection is most typically used to show the stereochemistry of alkanes since it focuses on a carbon-carbon bond.
A Newman projection depicts the front-to-back conformation of a chemical bond, with the front atom represented by the junction of three lines (a dot) and the back atom represented by a circle.
The front atom is referred to as proximal, whereas the back atom is referred to as distal. This style of representation vividly shows the dihedral angle formed by the proximal and distal atoms.
Newman projection formula
The molecules are viewed as superimposed circles in the Newman projection formula along the connection connecting the major carbon atoms. Short straight lines intersecting the center and circumference at an angle of 120° represent the remaining connections in each carbonyl group.
The center of the circle represents the front carbon atom, while the periphery represents the back carbon atom. The graphic below displays the Newman projections of ethane. There can only be one drawn circular.
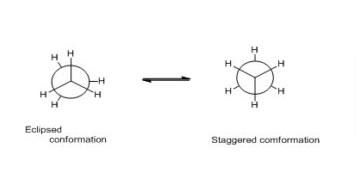
Newman projection examples
Newman projection of ethane
Newman projection of Ethane shows it has two conformations: staggered and eclipsed. The two ethane conformations, staggered and eclipsed, are distinct and should be in different energy levels.
Also Check For:
Also Check Relevant Topics like:
In eclipsed conformations, the hydrogen atoms on the front carbon overlap with the H atoms on the back carbon, causing repulsion between the electrons of the two carbons’ C-H bonds. Torsional strain, also known as eclipsing strain, is a form of repulsion. The Newman projection of ethane is mentioned below:
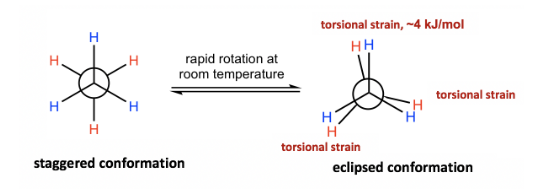
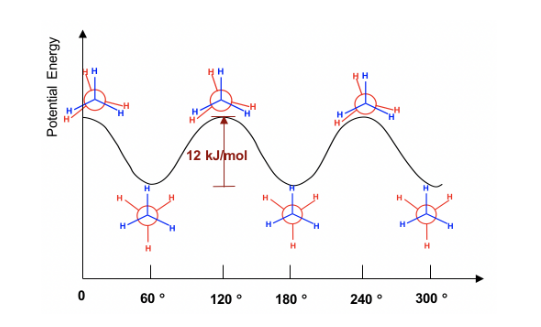
The Potential Energy of Newman projection of Ethane vs. Angle of the Rotation around a C-C bond is given below:
Newman projection of butane
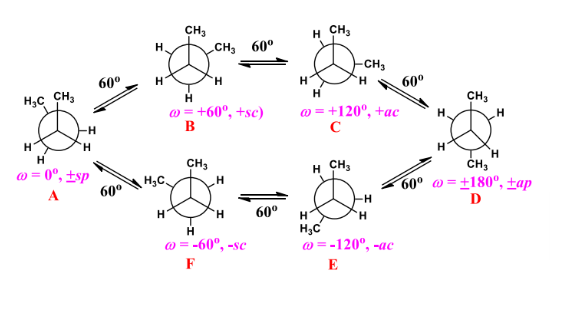
The rotation of the C2-C3 bond in butane in the first staggered conformation, the two methyl groups are 180° apart. This conformer is known as the anti conformer. In the opposite staggered conformer in the Newman projection of butane, both methyl groups flank each other.
A gauche contact occurs when two adjacent big groups with a staggered conformation interact. Gauche interactions enhance the amount of energy of a conformer. Because the staggered anti conformer had no gauche interactions. It has less energy compared to the staggered gauche conformed. The Newman projection of butane is shown below:
Cyclohexane newman projection
The most stable cycloalkane is cyclohexane. It is strain-free, which means there are no angle or torsional strains, and it has the same stability as chain alkanes. This exceptional stability is due to the peculiar conformation it assumes. The most stable cyclohexane conformation is known as the “chair” shape. The Newman projection of cyclohexane is shown below:
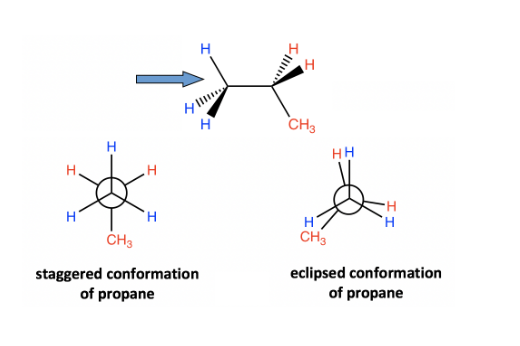
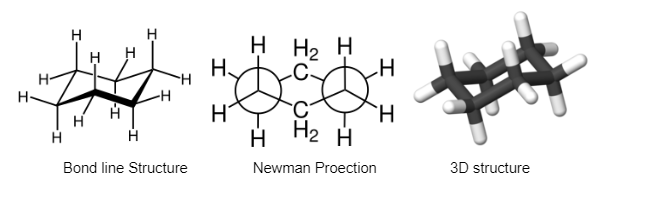 Newman projection propane
Newman projection propane
The distinction between propane and ethane is that propane has a methyl (CH3) group attached to the back carbon. However, the relative stability is unaffected, and the staggered conformer is more stable and has lower energy. The Newman projection of propane is shown below:
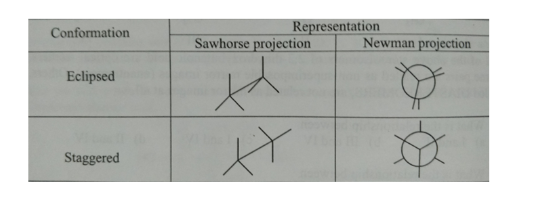
Newman and sawhorse projection
There are some differences in drawing Newman and sawhorse projection as shown below in the table:
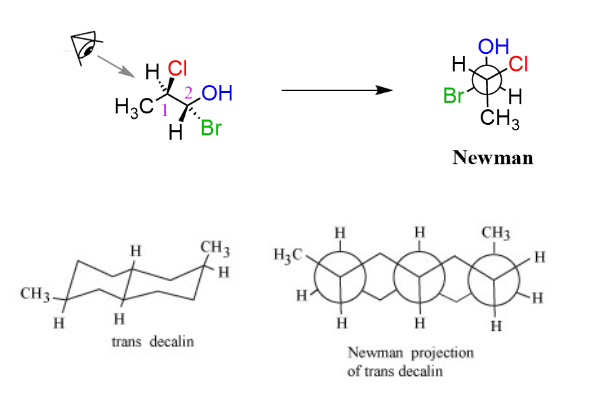
Complex newman projections
The Complex Newman projections of some of the molecules are given below:
Frequently Asked Questions on Newman Projections
What is Newman projection?
A Newman projection depicts the conformation of a chemical bond from front to back, with the line representing the front atom and a circle indicating the back carbon. It is useful in alkane stereochemistry. The carbon atom near the front is referred to as proximal, while the atom at the back is referred to as distal.
Draw newman projection formula of ethane.
Newman projection formula for several ethane conformations: When an ethane molecule spins around its carbon-carbon single bond, it can form two extreme conformations: the staggered conformation and the eclipsed conformation.
What is the best example of conformational changes?
Guanine and adenine nucleotide triphosphatases (NTPases) are two of the most well-known proteins that undergo large-scale conformational changes to regulate cell functions.
What is an example of a saw horse projection class 11?
A sawhorse projection is the appearance of a molecule with a certain Carbon-Carbon bond. Both the front and back carbon groups can be drawn with sticks at a 120-degree angle.








