Table of Contents
Introduction
Potassium dichromate is a chemical compound with significant importance in various industries, laboratories, and academic studies. Its unique properties and versatile applications make it a subject of interest for students studying chemistry.
This article aims to provide a comprehensive understanding of potassium dichromate, including its structure, formula, properties, preparation methods, and wide-ranging applications.
Potassium Dichromate Structure and Formula
The potassium dichromate formula is K2Cr2O7, representing two potassium (K) atoms, two chromium (Cr) atoms, and seven oxygen (O) atoms. The atoms are arranged in a specific structural pattern, forming dichromate ions (Cr2O72-) as follows in the following potassium dichromate structure:
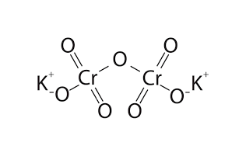
The arrangement of atoms determines the compound’s physical and chemical properties.
Potassium dichromate colour
Potassium dichromate exhibits a distinct bright orange-red color, which makes it easily recognizable. This vibrant color is a characteristic feature of potassium dichromate solutions and is attributed to the electronic transitions occurring within the compound.

Potassium Dichromate Preparation
Potassium dichromate can be prepared through different synthesis methods.
From Na2CrO4: The first stage yields a sodium chromate solution. This sodium chromate solution has been carefully purified using filtration to remove iron oxide and other contaminants. It is then treated with concentrated H2SO4, resulting in the conversion of sodium chromate to sodium dichromate.
2Na2CrO4 + H2SO4 → Na2Cr2O7 + Na2SO4 + H2O
From chrome iron ore: The ore is first pulverized and then combined with sodium carbonate, Na2CO3, and CaO (quick lime). It is then heated within a reverberatory boiler in the presence of adequate air. This step’s reaction can be written as:
4FeO.Cr2O3 + 8Na2CO3 + 7O2 → 8Na2CrO4 + 2Fe2O3 + 8CO2
Properties of Potassium Dichromate
Potassium dichromate exhibits distinct properties.
- Its physical properties include a non-combustible nature, solubility in water, and a density of approximately 2.68 g/cm³.
- The potassium dichromate molecular weight is 294 g/mol.
- Chemically, it acts as a strong oxidizing agent with a high oxidation potential, making it effective in various redox reactions.
Acidified Potassium Dichromate Formula
Acidified potassium dichromate, represented by the formula K2Cr2O7.H2SO4, is a modified form of potassium dichromate. It serves as a potent oxidizing agent in various chemical reactions, playing a crucial role in organic synthesis and other oxidation processes.
Chemical Reactions of Potassium Dichromate
Potassium dichromate (K2Cr2O7) is a versatile compound that undergoes various chemical reactions, each with its own unique outcomes and significance.
1. Potassium Dichromate + Sulphuric Acid
When potassium dichromate is combined with sulphuric acid (H2SO4), a redox reaction occurs. This reaction leads to the formation of chromium(III) sulfate (Cr2(SO4)3), water (H2O), and oxygen gas (O2).
2. Potassium Dichromate + Hydrochloric Acid
Mixing potassium dichromate with hydrochloric acid (HCl) triggers a similar redox reaction. The reaction produces chromium(III) chloride (CrCl3), water (H2O), and chlorine gas (Cl2).
3. Potassium Dichromate + Sulphur Dioxide
Reacting potassium dichromate with sulphur dioxide (SO2) yields chromium(III) sulfate (Cr2(SO4)3) and water (H2O). This reaction demonstrates the oxidizing properties of potassium dichromate, as it causes the conversion of sulphur dioxide to sulphate ions.
Potassium Dichromate Uses
Potassium dichromate finds extensive applications in both industrial and laboratory settings.
- Industrially, it is used in the production of dyes, pigments, and electroplating processes, enhancing the color and corrosion resistance of materials.
- In laboratories, it acts as an oxidizing agent in organic synthesis and is utilized in titration methods for determining the concentration of substances.
However, it is essential to consider the environmental implications and regulations associated with its use. Hence, there are numerous aspects where potassium dichromate uses have been seen.
Potassium Dichromate vs. Potassium Chromate and Potassium Permanganate
Potassium dichromate, potassium chromate, and potassium permanganate are three chemical compounds with distinct properties and applications. The potassium permanganate formula is KMnO4, whereas potassium dichromate is K2Cr2O7. Potassium dichromate (K2Cr2O77) undergoes a color change, while potassium permanganate (KMnO4) is known for its strong oxidizing properties.
Conclusion
Potassium dichromate, with its distinctive structure, formula, properties, preparation methods, and wide-ranging applications, is a compound of significant importance in the field of chemistry.
Understanding its properties and applications opens up avenues for further exploration and utilization. However, it is crucial to handle and use potassium dichromate responsibly, considering its potential hazards and environmental impact.
Frequently Asked Questions on Potassium Dichromate
Is K2Cr2O7 an acid or base?
Potassium dichromate, K2Cr2O7, is neither an acid nor a base. It is an ionic compound consisting of potassium cations (K+) and dichromate anions (Cr2O72-).
Why is K2Cr2O7 acidified?
K2Cr2O7, or potassium dichromate, is often acidified by adding sulfuric acid (H2SO4) during certain chemical reactions. Acidification helps to provide an acidic environment, which promotes the efficient oxidation reactions involving potassium dichromate.
Why is K2Cr2O7 an oxidizing agent?
Potassium dichromate acts as an oxidizing agent due to the presence of the dichromate ion (Cr2O72-). This ion has a high affinity for electrons and readily accepts them from reducing agents, leading to the oxidation of these substances. The transfer of electrons to the dichromate ion results in the reduction of chromium from a +6 oxidation state to a lower oxidation state.








