Table of Contents
Introduction to Schiff reagent
Schiff reagent (C19H21N3S2O7.4H2O) is a chemical formed by the reaction of sodium bisulfite and the magenta-colored dye fuchsin (C20H19N3.HCl).Because aldehydes can react with the decolorized Schiff reagent and restore their original color, it is often employed as a chemical test to detect the presence of aldehydes in a sample.
Hugo Schiff, a German scientist, discovered Schiff Reagent. Schiff’s reagent was used for the first time in 1897. For the first time in a biological study, Feulgen used Schiff’s reagent in 1924.
The staining process was later named after him. To identify aldehydes formed by the acidic breakdown of deoxyribose sugar in deoxyribonucleic acid (DNA), Feulgen used Schiff’s reagent.
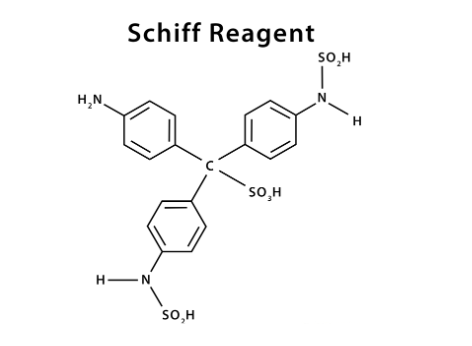
Schiff reagent formula
The Schiff reagent formula is C19H21N3S2O7.4H2O. The Schiff reagent structure is shown below:
Schiff’s reagent Chemical Name
Schiff’s reagent chemical name is p-rosaniline hydrochloride solution.
Schiff’s reagent name
Schiff reagent is a byproduct of the chemical reaction of certain dyes, such as fuchsin and sodium bisulfate, with aldehydes to produce a vivid pink result. Schiff reagent is also known as leucofuchsin.
Schiff reagent colour
Schiff reagent colour is white. The presence of aldehydic and ketonic groups is detected using Schiff’s reagent. It is made out of fuchsin dye that has been decolored by sulphuric acid. The presence of aliphatic aldehyde is detected by its immediate red/pink color.
It takes time for aliphatic ketones and aromatic aldehydes to bloom, and the pink color emerges gradually.
Schiff reagent composition
Schiff reagent composition made by combining 1% fuchsin dye in water (>98%) with 1% sodium bisulfite mixed in a 1% hydrochloric acid solution. Before being decolored with charcoal, the solution is agitated at regular intervals.
After that, the mixture is filtered. Use freshly activated charcoal to ensure the formation of a colorless solution. The solution is filtered again if it is not colorless.
Schiffs reagent preparation
The schiffs reagent preparation procedure is as follows.
- In 900 ml of boiling water, dissolve 5g of basic fuchsin. Allow the solution to cool to a temperature of roughly 50 degrees Celsius.
- Add 100 cc of 1M HCl at a time to the diluted fuchsin solution.
- Reduce the solution’s temperature to 25 degrees Celsius. To the cooled solution, add 10g of NaHSO3.
- Shake the solution for 3 minutes before incubating it in the dark for 24 hours.
- After incubation, stir in 5g of fine activated charcoal. After 3 minutes of shaking, strain the solution.
- If a clear solution is not obtained, repeat the filtration and treatment process.
- To prevent the precipitation of white crystalline material, store the prepared solution in a foil-covered vial at 4 degrees Celsius.
- To achieve reliable findings, fresh batches of Schiff Reagent should be prepared every 2-3 weeks.
Schiffs reagent Test
When an unknown aldehyde solution is introduced to the colorless Schiff reagent, it returns to its previous magenta color. This is referred to as the Schiff reagent test.
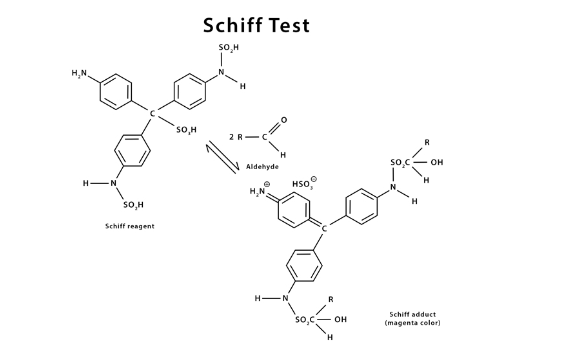
The Schiff test dates back to 1866, when German-Italian scientist Hugo Schiff invented it and published it in a magazine.
Also Check For:
Schiff reagent reaction with aldehyde
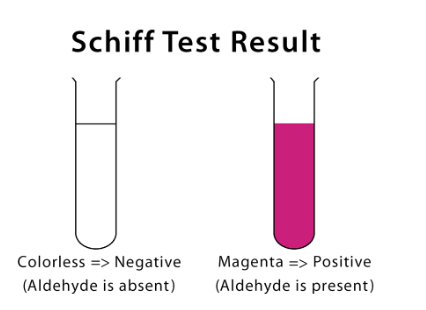
The Schiff reagent gives pink colour when aldehyde is present as shown below.
Schiff base reagent
Schiff base reagent is N-alkyl diamine which is a result of the reaction of aldehydes or ketones with primary amines.
Difference between Schiff base and Schiff reagent
The primary difference between Schiff base and Schiff reagent is that the former relates to either secondary ketimines or secondary aldimines, whereas the latter refers to a reagent used to test for aldehydes and ketones. Hugo Schiff inspired the names of Schiff base and Schiff’s reagent.
Frequently Asked Questions on Schiff reagent
What is schiff's reagent?
Schiff's Reagent is an aqueous solution of magenta or pink-colored Rosaniline Hydrochloride that decolorizes when SO2 is run through it, which is why it is employed as an aldehyde differentiation test.
How is schiff's reagent prepared?
Schiff reagent is made by combining 1% fuchsin dye in water (>98%) with 1% sodium bisulfite mixed in a 1% hydrochloric acid solution. The solution is shaken at regular intervals before being decolored with charcoal.
What is the reaction of Schiff's reagent?
When Schiff's reagent reacts with acetaldehyde, it produces a pink color. Paul Rumpf (1908-1999) postulated an imine-mediated mechanism in 1935, and Hardonk and van Duijn presented experimental evidence in 1964.
What is the name of Schiff's reagent?
p-rosaniline hydrochloride solution. Schiff's solution is a sulphurous acid-treated p-rosaniline hydrochloride solution.
Why is Schiff base called base?
The phrase Schiff's base comes from the name of German chemist Hugo Schiff, who was the first to characterize the results of the interaction of primary amines with carbonyl compounds in 1864.








