Table of Contents
Dual Nature of Matter and Light: Albert Einstein (1879-1955) proposed a theory in 1905 that claimed light has a dual nature. Light has to have the attributes of both a wave and a particle. A photon is a quantum of energy that is connected with each particle of light. Planck’s equation can be used to calculate the energy of a photon. The light emitted when current is transmitted through a gas in a cathode ray tube was explained thanks to Einstein’s hypothesis. The ground state describes an atom with the lowest attainable potential energy. The potential energy of the atoms increases when a current runs through a gas at low pressure. An atom in an excited state has higher potential energy than just its ground state.
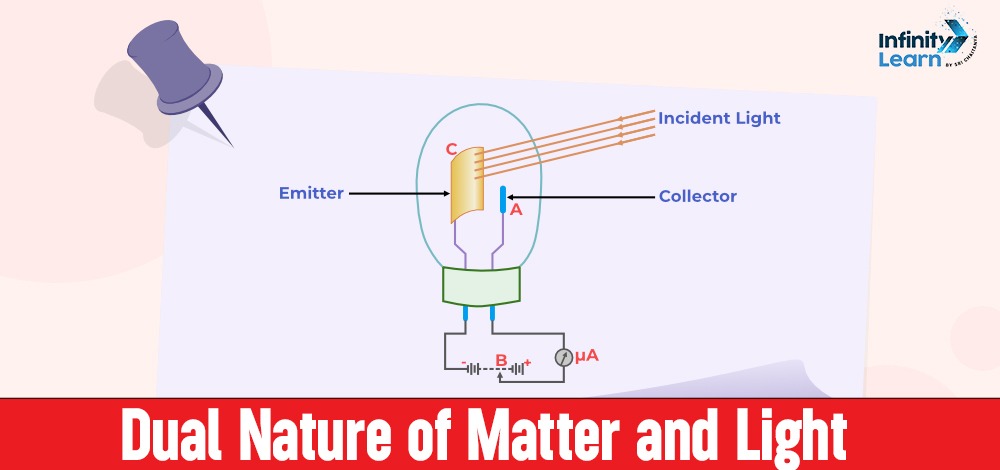
Dual Nature of Matter and Light – A brief outline
A particle is a concentration of energy and other qualities in space and time, even though a wave is spread out over a greater region of space and time, according to the classical perspective. The debate over whether light is made up of streams of particles (corpuscles) or waves has been going on for a long time. This “either-or” formulation was natural in the past and is now alien to advanced “both-and” and even “neither-nor” solutions. Experiments were proposed and carried out in the early nineteenth century to demonstrate that light has a wave motion.
Thomas Young, one of the most bright and clever scientists ever to live, was a significant figure in this effort. He examined diffraction & interference of light in 1803 and came up with data that backed up Christian Huygens’ wave theory over Isaac Newton’s particle or corpuscular theory. Many other researchers contributed, including Augustin Jean Fresnel, who demonstrated that light is a transverse wave. According to the de Broglie notion of matter waves, the matter has a dual nature, which implies that when it is moving, it exhibits wave properties (such as interference, diffraction, and so on) and when it is at rest, it exhibits particle properties. As a result, the matter has a dual character.
Important concepts
Dual nature of matter and light
Lewis de-Broglie postulated in 1924 that matter, like radiation, has a dual nature. His theory of matter’s dual nature was founded on the following observations: –
(a) Matter and electromagnetic radiations make up the entire universe. Because they are both types of energy, they can be converted into one another.
(c) Symmetry appeals to matter. Because radiation has a dual nature, the matter should have a dual nature as well.
The matter has a twofold character, as per the de Broglie idea of matter waves. It means that when the matter is moving, wave qualities (such as interference, diffraction, and so on) are connected with it, and when it is at rest, particle attributes are associated with it. As a result, the matter has a dual character. Matter waves, also known as de-Broglie waves, are related to moving particles.
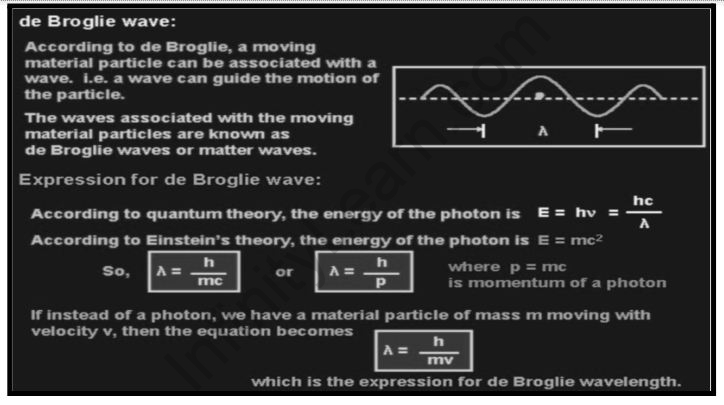
THE DE-BROGLIE WAVELENGTH
Consider a photon with the following energy:
E=hυ=hc/λ – – – (1)
If a photon has mass (its rest mass is zero), then its energy is provided by according to the theory of relativity.
E=mc2 – – – (2)
We have (1) and (2)
m=h/c is the mass of a photon
As a result, photon momentum
P=mc=hc/cλ=h/λ – – – (3)
Alternatively, λ = h/p
If we assume a material particle of mass m travelling with velocity v instead of a photon, the momentum of the particle is p=mv. As a result, the wavelength of the wave related to this flowing particle can be calculated as follows:
h/mv
Alternatively, λ = h/p (where p = mv) (4)
The DE-Broglie wavelength is the name given to this wavelength.
In quantum physics, wave-particle duality goes on to state that any particle, as well as a quantum phenomenon, can be characterized as either a particle or a wave. It expresses the difficulty of classical terminology like “particle” and “wave” to completely characterize quantum-scale things’ behavior. The present scientific hypothesis states that all particles have a wave nature and vice versa, according to the contributions of Max Planck, Albert Einstein, Louis de Broglie, Arthur Compton, Niels Bohr, Erwin Schrödinger, and others. This phenomenon has been confirmed for both elementary and compound particles, such as atoms and molecules. Wave characteristics are generally not detectable for large particles due to their extremely particular wavelengths.
Dual Nature of Radiation
The particle-like behavior is still most obvious due to quantum mechanics phenomena connected with measurement. The uncertainty principle dictates that when a particle’s position is measured, the particle is driven into a more localized state. The measurement of the wave function, when viewed via this formalism, will result in the wave function collapsing to a sharply crested function at some point. The probability of detecting a particle with mass at any given point is equal to the squared amplitude of the wave function here. Heisenberg’s uncertainty principle applies to the measurement, which will yield a well-defined position.
The standard model and the de Broglie–Bohr theory are two approaches to visualize the wave-particle behaviour. The more localized the position-space wavefunction, the more probable the particle will be located with position coordinates in that region, and the momentum-space wavefunction, similarly, is less localized, resulting in a wider range of possible momentum components for the particle. Conversely, the more localized the momentum-space wavefunction, the more likely the particle will be discovered in that region with those values of momentum components, and the less localized the position-space wavefunction, the more widespread the position coordinates the particle may occupy.
Frequently asked questions
What is the meaning of wave-particle duality?
In quantum physics, wave-particle duality states that any particle or quantum phenomenon can be characterized as either a particle or a wave. It expresses the incapacity of classical ideas like particle and wave to completely characterize quantum-scale things' behaviour. Light has the properties of both a wave and a particle, according to wave-particle theory.
What is the procedure that indicates the light is a wave?
Atomic absorption and emission, the photoelectric effect.
What is Black Body Radiation, and how would it work?
An ideal black body is a perfect radiation absorber and emitter. When a body like this is heated, it turns red. To put it another way, it emits a reddish light. As the temperature rises, the colour of the radiated radiation shifts from red to yellow to white, then purple as the temperature rises even higher. This means that when the temperature rises, the wavelength of the radiation emitted by a dark body decreases.
Infinity Learn App
Now you can find answers to all your subject queries & prepare for your Exams on our Ultimate learning app for classes 3 to 12 – Infinity Learn.









