Table of Contents
What is called lipid?
Lipids are a group of fatty organic compounds insoluble in water. The other property of lipids is that they are soluble in nonpolar solvents such as alcohol, ether, and chloroform. Fats, oils, waxes, phospholipids, and steroids are important components of living cells.
They are part of the cell membranes and play a role in controlling what enters and exits the cells. They help in energy movement and storage, vitamin absorption, and hormone production.
Lipids Definition in Biochemistry
In biochemistry, lipid is defined as naturally occurring molecules that include fats, oils, and waxes. The composition of lipids includes carbon, hydrogen, and oxygen. Lipids are important as they store energy, act as signaling molecules in the body, and form cell membranes.
Since lipids are insoluble in water, they form protective barriers, such as the outer layer of cells. Common types of lipids include-
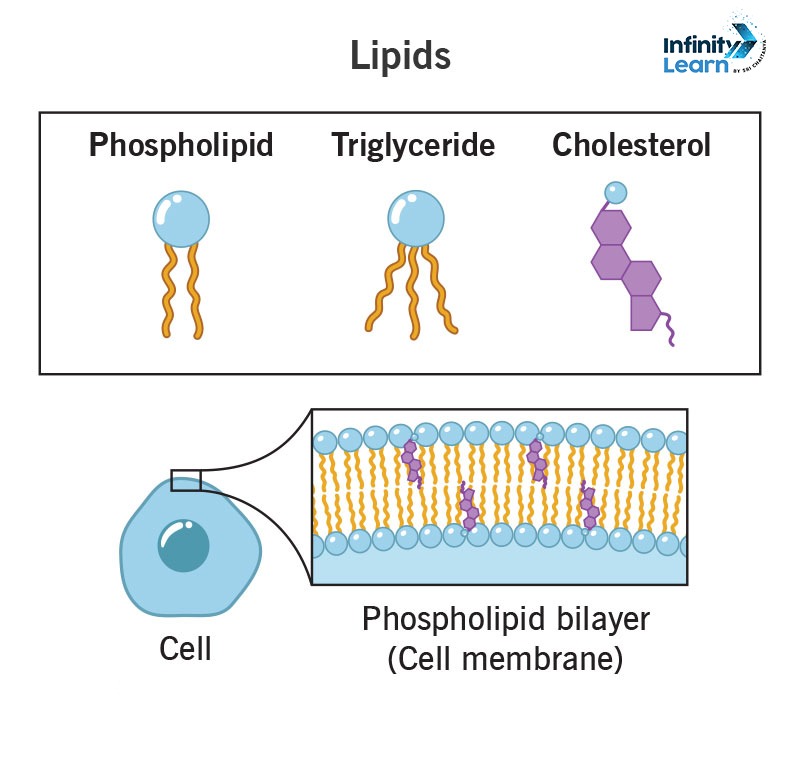
- Triglycerides (fats and oils)
- Phospholipids (in cell membranes)
- Steroids (like cholesterol and hormones)
Let us discuss the types of lipids in detail going further.
Classification of Lipids
Lipids are classified into different categories based on their structure and function. Here are the types of lipids discussed below.
- Simple lipids (Fats and Waxes)
- They are also known as triglycerides.
- Composed of glycerol and three fatty acid chains.
- Fats are solid at room temperature, while oils are liquid.
- Waxes are esters of long-chain fatty acids with long-chain alcohols.
- They are used by plants and animals for protection and waterproofing.
- Complex Lipids
- They include phospholipids, glycolipids, and lipoproteins.
- Phospholipids contain a phosphate group, glycerol, and two fatty acids.
- Glycolipids are carbohydrate groups that are attached to a lipid.
- Lipoproteins are complexes of lipids and proteins that transport fats in the blood (e.g., HDL, LDL).
- Derived lipids
- They are those lipids that are formed after the breakdown of simple and complex lipids.
- They have a specific role in the body and are not found in the free form.
- These lipids include substances that have characteristics of lipids but are structurally different from fats or oils.
- They are considered as the intermediates or products of lipid metabolism.
- Examples include – Steroids, fatty acids, monoglycerides, diglycerides, and fat-soluble vitamins.
Properties of Lipids
Lipids have a wide range of physical and chemical properties. Here are the important properties exhibited by the lipids mentioned below.
- Hydrophobicity
- Lipids being hydrophobic, are non-polar and hydrophobic. This means that they are insoluble in water.
- Hydrophobicity allows cell membranes to form barriers, allowing selective passage of nutrients.
- Lipids dissolve in organic solvents like – ether, benzene or chloroform.
- Energy Storage
- Lipids store more energy as compared to proteins and carbohydrates.
- This makes them energy reservoirs for organisms.
- Amphipathic nature
- When lipids show dual nature, which means showing both water-fearing and water-attracting features.
- This allows them to form bilayers in cell membranes.
- In this, the hydrophobic tails face inward and the hydrophilic heads face outward.
- Melting point
- Saturated fats have lower melting point, and are solid at room temperature.
- Unsaturated fats have lower melting points, and are liquid at room temperature.
- Saponification
- Lipids like triglycerides are hydrolyzed with an alkali like NaOH.
- They undergo saponification, producing soap and glycerol.
- Emulsification
- They can form emulsions when dispersed in water.
- It is done by using emulsifiers such as bile salts.
What is the structure of Lipid?
Lipids are generally made of hydrocarbons, but their structure differ depending on the types. Different lipids have different structure, here’s the overview of each structure.
- Triglycerides
- They are oils and fats, composed of one glycerol molecule (3-carbon alcohol) and three fatty acid chains.
- Each fatty acid is a long hydrocarbon chain with a carboxyl group (-COOH) at one end.
- The fatty acids are attached to the glycerol via ester bonds through a dehydration reaction.
- There are three fatty acids attached to the glycerol backbone. They can be saturated (single bonds) or unsaturated (double bonds).
- Phospholipid
- In this, two fatty acids are attached to a glycerol backbone.
- Third hydroxyl group of glycerol is attached to a phosphate group (PO₄³⁻).
- This is often linked to other polar groups (such as choline or serine).
- Steroids
- Characterised by a core structure of four fused carbon rings
- It has three cyclohexane rings and one cyclopentane ring.
Lipids FAQs
What are lipids, and why are they important?
Lipids are a diverse group of organic compounds that are insoluble in water but soluble in nonpolar solvents. They play crucial roles in the body, including storing energy, forming cell membranes, and acting as signaling molecules.
What is the basic structure of lipids?
Lipids typically consist of a glycerol backbone bonded to fatty acids. The structure can vary, leading to different types of lipids, such as triglycerides, phospholipids, and sterols, each with specific functions in the body.
How are lipids classified?
Lipids are classified into several categories: simple lipids (like fats and oils), complex lipids (such as phospholipids), and derived lipids (including sterols and fat-soluble vitamins). Each category has different structures and functions in biological systems.
What are the properties of lipids that make them unique?
Lipids are characterized by their hydrophobic nature, meaning they do not mix well with water. They are also amphipathic, with both hydrophobic and hydrophilic parts, allowing them to form structures like cell membranes.
How do lipids function in the human body?
Lipids serve several essential functions, including energy storage, structural components of cell membranes, insulation and protection of organs, and acting as precursors for hormones and other signaling molecules.









