Table of Contents
Benzoic Acid – Structure and Properties
Benzoic acid is an organic compound with the chemical formula C₆H₅COOH. It belongs to the carboxylic acid family and is derived from benzene. It is a white crystalline solid with a characteristic odor.
Benzoic acid has been known and used for centuries. It occurs naturally in various fruits, particularly berries, and is also found in many plants as a metabolic product. In its natural form, it acts as a preservative, inhibiting the growth of bacteria and fungi.

What is the Structure Of Benzoic Acid?
Benzoic acid is a white crystalline solid with the chemical formula C7H6O2. Its molecular structure consists of a benzene ring (a six-carbon aromatic ring) with a carboxylic acid functional group (-COOH) attached to one of the carbon atoms in the ring. The carboxylic acid group consists of a carbonyl group (C=O) and a hydroxyl group (-OH) attached to the same carbon atom. The molecular formula and structure of benzoic acid can be represented as follows:
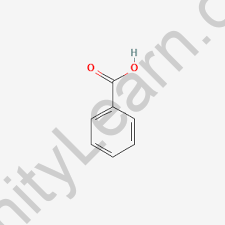
The benzene ring is flat and planar, with each carbon atom bonded to two adjacent carbon atoms and one hydrogen atom. The carboxylic acid group is attached to the benzene ring at the first carbon atom (also called the alpha carbon) and extends out of the plane of the ring. The carboxylic acid group is responsible for the acidic properties of benzoic acid.
What are the Physical Properties of Benzoic Acid?
The physical properties of benzoic acid include:
- State: Benzoic acid is a white crystalline solid at room temperature.
- Odor: It has a characteristic sweet, somewhat musty odor.
- Melting Point: The melting point of benzoic acid is relatively high, around 121-123 degrees Celsius (250-253 degrees Fahrenheit). This makes it a solid at room temperature.
- Boiling Point: Benzoic acid has a boiling point of approximately 249 degrees Celsius (480 degrees Fahrenheit).
- Solubility: It is sparingly soluble in cold water, but its solubility increases with temperature. It is more soluble in organic solvents such as ethanol, ether, and chloroform.
- Density: The density of benzoic acid is about 1.32 grams per cubic centimeter.
- Crystal Structure: Benzoic acid crystals typically exhibit a monoclinic crystal structure.
- pH: Benzoic acid is an acidic compound, and in aqueous solutions, it exhibits a pH below 7.
- Vapor Pressure: The vapor pressure of benzoic acid is relatively low, meaning it has a low tendency to evaporate at normal temperatures.
- Appearance: It appears as white or colorless crystals or powder.
These physical properties play a role in its various applications, such as its use as a preservative, its solubility in different solvents, and its behavior during heating or cooling processes.
What are the Chemical Properties of Benzoic Acid?
Benzoic acid exhibits several notable chemical properties:
- Acidic Nature: Benzoic acid is a weak acid and can donate a proton (H+) from the carboxylic acid group (-COOH) in solution. It dissociates partially, releasing hydrogen ions. The equilibrium constant for this dissociation is represented by the acid dissociation constant (pKa) of benzoic acid, which is approximately 4.2. The carboxylic acid group is responsible for its acidic properties.
- Solubility: Benzoic acid is sparingly soluble in water at room temperature. However, it dissolves more readily in hot water or in the presence of a suitable solvent, such as alcohol or ether.
- Reaction with Bases: Benzoic acid reacts with bases to form salts known as benzoates. When a strong base, such as sodium hydroxide (NaOH), is added to benzoic acid, sodium benzoate is formed, accompanied by the release of water.
C6H5COOH + NaOH → C6H5COONa + H2O
- Esterification: Benzoic acid can undergo esterification reactions with alcohols in the presence of an acid catalyst. This reaction forms esters, which are compounds with the general formula RCOOR’. This process is often used in the synthesis of various benzoate esters.
C6H5COOH + R’OH → C6H5COOR’ + H2O
- Oxidation: Benzoic acid can be oxidized to form benzaldehyde or further oxidized to benzoic acid derivatives. Strong oxidizing agents, such as potassium permanganate (KMnO4) or chromic acid (H2CrO4), can be used for this purpose.
- Decarboxylation: Under certain conditions, benzoic acid can undergo decarboxylation, where the carboxyl group (-COOH) is removed, resulting in the formation of benzene. This reaction usually requires high temperatures or the presence of a catalyst.
These are some of the notable chemical properties of benzoic acid. Its unique properties make it useful in various applications, including as a food preservative, flavoring agent, and intermediate in organic synthesis.
Formation of Salts
- Salts are ionic compounds composed of a metal cation and a nonmetal anion. The metal cation and the nonmetal anion are held together by electrostatic forces.
- When a salt is formed, the metal cation and the nonmetal anion exchange electrons. This process produces a stable ionic compound.
- The metal cation and the nonmetal anion are both neutral molecules before the salt is formed. However, the salt molecule has a net charge of +1 because of the positive charge of the metal cation and the negative charge of the nonmetal anion.
Formation of Esters
- When an alcohol and a carboxylic acid react, the alcohol group (OH) attaches to the carboxylic acid group (COOH) to form an ester.
- The ester is a water-soluble molecule that is generally less acidic than the original acid and alcohol.
Formation of Acid Halides
Acid halides are formed when an acid and a halogen react. In this reaction, the haloge
Sulfonation
Sulfonation is a chemical reaction that introduces a sulfonic acid group (-SO3H) into an organic molecule. The sulfonic acid group is a strong acid that can dissociate in water to form sulfuric acid. Sulfonation is used to produce detergents, pharmaceuticals, and other products.
Nitration of aniline
- The nitration of aniline proceeds through the intermediary of a nitronium ion. The nitronium ion is a strong electrophile that is formed when nitric acid and aniline are mixed.
- The electrophile attacks the electron-rich aromatic ring, displacing a hydrogen atom. This forms a new carbon-nitrogen bond and a nitro group.
Halogenation
Halogenation is a chemical reaction that involves the addition of one or more halogens to a molecule. The most common halogens are fluorine, chlorine, bromine, and iodine.
Inhalation
Inhalation is the process of breathing in air or other gases.
Decarboxylation of Benzoic Acid
- The first step in the synthesis of benzocaine is the decarboxylation of benzoic acid. In this step, the carboxylic acid group is removed from the molecule, and a benzene ring is formed.
- The reaction is catalyzed by a base, and it can be done in either acidic or basic conditions. In acidic conditions, the carboxylic acid group is protonated, and in basic conditions, the carboxylic acid group is deprotonated.
- The most common base used for this reaction is sodium hydroxide, but other bases such as potassium hydroxide can also be used.
Reduction of Benzoic Acid to Benzene
- In the presence of a strong base, benzoic acid can be deprotonated to form the benzoate anion. The benzoate anion can then be reduced to benzene using a reducing agent.
- Benzene is a colorless, flammable liquid with a sweet odor. It is primarily used as a precursor to other chemicals.
Dehydration Reaction
A dehydration reaction is a chemical reaction in which water is removed from a molecule. The most common type of dehydration reaction is the dehydration of alcohols to form alkenes.
Reduction of Benzoic Anhydride to Benzoic Acid
- The reduction of benzoic anhydride to benzoic acid can be achieved through the use of a metal catalyst, such as palladium on carbon, or a metal oxide, such as nickel oxide. The reaction is typically conducted in an aprotic solvent, such as dimethylformamide (DMF), at a temperature of around 100°C.
- The overall reaction can be represented as follows:
- The reduction of benzoic anhydride to benzoic acid can also be conducted using a heterogeneous catalyst, such as palladium on charcoal.
Controlled Oxidation of Benzyl Alcohol
- Benzyl alcohol is oxidized to benzoic acid in the presence of an oxidizing agent, such as potassium permanganate.
- The oxidation of benzyl alcohol to benzoic acid can be represented by the following equation:
- 2 C6H5CH2OH + KMnO4 → 2 C6H5COOH + MnO2 + H2O
Uses of Benzoic Acid
- Benzoic acid is used to make a preservative called benzoic acid. Benzoic acid is also used to make a dye called benzopyrene.
- Benzoic acid is a colorless crystalline solid that is soluble in water and has a characteristic odor.
- It is used in the production of plastics, resins, and dyes. Benzoic acid is also used as a food preservative and as a fungicide.









