Table of Contents
Respiration is a fundamental process that fuels life by extracting energy stored in molecules like NADH+H+ and FADH2. This energy is harnessed through a complex sequence of events known as the Electron Transport Chain, leading to the production of ATP, the cellular energy currency. In this article, we will explore the intricacies of the electron transport Chain, its various components, electron carriers, and the role of oxygen as the terminal electron acceptor.
Electron Transport Chain in Respiration
The electron transport chain, often referred to as the electron transport system (ETS), is a critical component of cellular respiration. It is located within the inner mitochondrial membrane, where it plays a pivotal role in the generation of ATP, the energy source required for various cellular activities.
The Various Complexes Involved and Their Composition
The ETC consists of several complexes, each with specific functions and unique compositions:
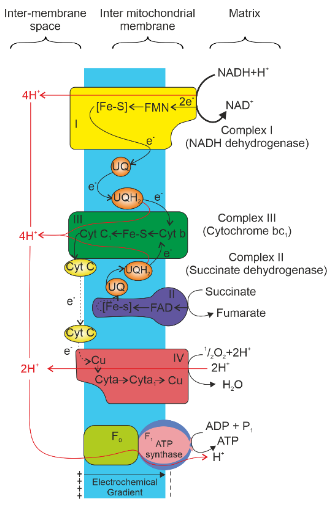
Complex I (NADH Dehydrogenase Complex)
Major Component: Flavoprotein containing flavin mononucleotide (FMN), Iron-sulphur protein (FeS), and NADH binding site.
Complex II (Succinate Dehydrogenase)
Major Component: Iron-sulphur protein and FADH2 binding site.
Complex III (Cytochrome bc1 Complex)
Major Component: Cytochrome b, Cytochrome c1, and Rieske iron-sulphur protein.
Complex IV (Cytochrome c Oxidase Complex)
Major Components: Cytochrome a, Cytochrome a3, and two copper centres.
The Various Electron Carriers
- Ubiquinone (Coenzyme Q): A mobile electron carrier located within the inner membrane, ubiquinone receives electrons from both complex I and II.
- Cytochrome c: This mobile carrier shuttles electrons between complex III and complex IV.
Pathway of Electron Transport
The inner mitochondrial membrane, characterized by its folded structures called cristae, plays host to the ETC in eukaryotic cells. This folding dramatically increases the membrane’s surface area, allowing for the installation of thousands of ETC copies within each mitochondrion. Here, the principle of structure fitting function becomes evident. The intricate alignment of electron carrier molecules in a sequential row within the infolded membrane perfectly suits the series of redox reactions that transpire along the ETC.
The journey of electrons commences when NADH, a product of glycolysis and the citric acid cycle, donates its electrons to complex I. This first molecule in the chain is a flavoprotein, named for its prosthetic group, flavin mononucleotide (FMN). In the subsequent redox reaction, the flavoprotein returns to its oxidized state while passing electrons to an iron-sulphur protein (FeS in complex I), a family of proteins with tightly bound iron and sulphur atoms. The baton is then passed to ubiquinone (Q), also known as coenzyme Q (CoQ) is a small hydrophobic molecule and the sole nonprotein member of the ETC. Ubiquinone is uniquely mobile within the membrane, residing independently rather than within a specific complex.
As electrons journey from ubiquinone toward their final destination, oxygen, they traverse a series of proteins called cytochromes. These proteins boast prosthetic groups known as haem groups, featuring iron atoms that readily accept and donate electrons. Within the ETC, multiple types of cytochromes exist, each harbouring a distinct haem group tailored for electron transport.
The ultimate cytochrome, Cyt a3, delivers its electrons to oxygen, a highly electronegative molecule. This interaction results in the capture of a pair of hydrogen ions (protons) from the surrounding aqueous solution, effectively neutralizing the -2 charge associated with the added electrons and giving rise to water molecules.
Another contributor of electrons to the ETC is FADH2, the alternative reduced product produced during the citric acid cycle. FADH2 injects its electrons into the ETC from within complex II, at a lower energy level compared to NADH.
Although the electron transport chain does not directly produce ATP, it plays a crucial role in facilitating the transfer of electrons from food molecules to oxygen. This process involves breaking down a substantial free-energy drop into a series of smaller, manageable steps, each releasing energy.
The Terminal Electron Acceptor
The significance of oxygen in respiration lies in its role as the terminal electron acceptor. Oxygen captures electrons from complex IV, forming water (H2O). This final step ensures the continued flow of electrons through the electron transport chain, driving the synthesis of ATP.
Conclusion
The electron transport Chain is a pivotal player in cellular respiration, allowing cells to efficiently convert stored energy into ATP. This process, known as oxidative phosphorylation, relies on a series of complexes, electron carriers, and the ultimate acceptance of electrons by oxygen. Understanding these intricacies sheds light on the fundamental energy-generating processes that sustain life.
Frequently Asked Questions on Electron Transport Chain
What is the electron transport system in respiration?
The electron transport system (ETS) is a crucial component of cellular respiration located in the inner mitochondrial membrane. It facilitates the transfer of electrons from molecules like NADH and FADH2 to oxygen, resulting in the production of ATP.
What are the main complexes involved in the electron transport system?
The ETS consists of several complexes, including Complex I (NADH Dehydrogenase), Complex II, Ubiquinone (Coenzyme Q), Complex III (Cytochrome bc1 Complex), Cytochrome c, and Complex IV (Cytochrome c Oxidase).
What are the key electron carriers in the electron transport system?
Key electron carriers include ubiquinone, and cytochrome c. These carriers transport electrons between complexes in the ETS.
What is the role of oxygen in the electron transport system?
Oxygen serves as the terminal electron acceptor in the ETS, capturing electrons from Complex IV to form water. This step is crucial for maintaining the flow of electrons through the chain and ATP synthesis.
What is oxidative phosphorylation?
Oxidative phosphorylation is the process in which the energy released during electron transport is utilized to synthesize ATP. It involves the coupling of electron transfer with proton pumping to create an electrochemical proton gradient used to produce ATP.
How is ATP synthesized in the electron transport system?
ATP synthesis in the ETS occurs through ATP synthase (Complex V), which utilizes the proton gradient created during electron transport to convert ADP and inorganic phosphate into ATP. The passage of protons through ATP synthase powers this process.









