Table of Contents
What is osmosis?
Osmosis is the spontaneous movement of solvent molecules (typically water) across a selectively permeable membrane from an area of lower solute concentration to an area of higher solute concentration, aiming to equalize the concentration on both sides of the membrane.
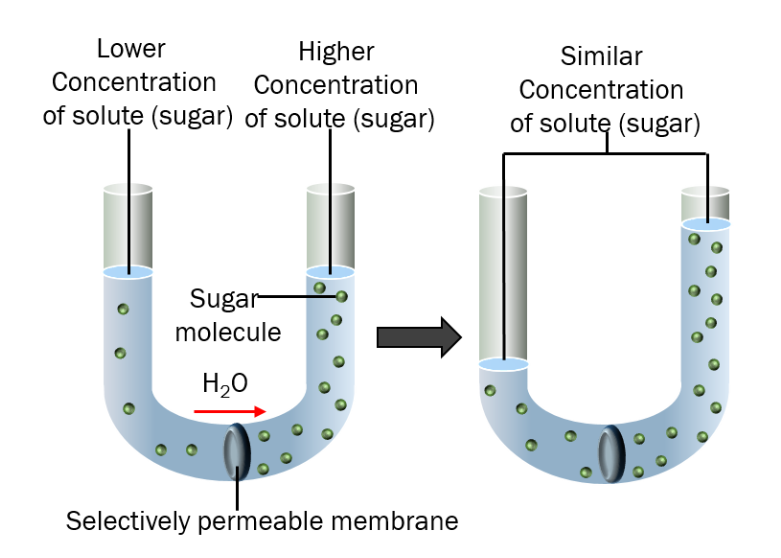
Brief explanation of Osmosis
Osmosis is the movement of solvent molecules, typically water, across a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration. It plays a vital role in various biological processes, such as maintaining cell hydration and facilitating nutrient uptake in plants and animals. Osmosis is driven by the concentration gradient and does not require external energy input.
Types of osmotic solutions
There are three main types of osmotic solutions based on the relative concentrations of solute and solvent:
Hypertonic Solution: In a hypertonic solution, the solute concentration is higher outside the cell or across a membrane compared to the inside of the cell. As a result, water tends to move out of the cell, causing it to shrink or undergo crenation.
Hypotonic Solution: In a hypotonic solution, the solute concentration is lower outside the cell or across a membrane compared to the inside of the cell. Water tends to move into the cell, causing it to swell or potentially burst in a process called lysis.
Isotonic Solution: In an isotonic solution, the solute concentration is equal both outside the cell or across a membrane and inside the cell. There is no net movement of water, and the cell remains in a stable state without significant changes in size or shape.
Types of osmosis
Exosmosis and endosmosis are terms that were historically used to describe osmosis in the context of plant cells and solutions.
Exosmosis
Exosmosis refers to the outward flow of water from a cell or plant tissue due to osmosis. It was used to describe the movement of water from the interior of the cell to the external environment when the external solution had a higher solute concentration compared to the cell.
Endosmosis
Endosmosis is used to describe the inward flow of water into a cell or plant tissue due to osmosis. It described the movement of water from the external environment into the cell when the external solution had a lower solute concentration compared to the cell.
Effect of osmosis on plant cells
Osmosis plays a significant role in plant cells and has various effects depending on the concentration of solutes inside and outside the cell. Here are two primary effects of osmosis on plant cells:
Turgor Pressure: When a plant cell is placed in a hypotonic solution (lower solute concentration outside the cell), water enters the cell through osmosis. This uptake of water creates internal pressure against the cell wall, resulting in turgor pressure. Turgor pressure provides structural support to the plant, helps maintain cell shape, and enables the plant to remain upright.
Plasmolysis: In contrast, when a plant cell is placed in a hypertonic solution (higher solute concentration outside the cell), water tends to leave the cell through osmosis. As water exits, the cytoplasm shrinks away from the cell wall, resulting in plasmolysis. Plasmolysis causes the cell to become flaccid and can lead to wilting or even cell death if prolonged.
The balance between these effects is crucial for the overall health and functioning of plant cells. Proper osmotic regulation is essential for maintaining cell turgidity, facilitating nutrient uptake, and ensuring appropriate water balance in plants.
Effect of osmosis on animal cells
Osmosis also has significant effects on animal cells, impacting their structure and function. Here are two main effects of osmosis on animal cells:
Cell Swelling or Bursting: When an animal cell is placed in a hypotonic solution (lower solute concentration outside the cell), water enters the cell through osmosis. This influx of water causes the cell to swell and potentially burst, a process known as cytolysis or cell lysis. The lack of a rigid cell wall in animal cells makes them more susceptible to bursting under hypotonic conditions.
Cell Shrinkage or Shrinking: Conversely, when an animal cell is placed in a hypertonic solution (higher solute concentration outside the cell), water tends to leave the cell through osmosis. As water exits, the cell shrinks and becomes dehydrated. This process is known as crenation and can impair the cell’s function and integrity.
Osmotic pressure
Osmotic pressure is the pressure exerted by a solvent, typically water, as it moves across a semipermeable membrane to equalize the solute concentrations on both sides. It is a colligative property, meaning it depends solely on the concentration of solute particles.
When there is a difference in solute concentration between two compartments separated by a semipermeable membrane, water molecules move from the side with lower solute concentration to the side with higher solute concentration. This movement continues until the osmotic pressure of the solution on the side with higher solute concentration is sufficient to prevent further net movement of water.
The osmotic pressure is directly proportional to the concentration of solute particles in the solution. Higher solute concentration results in higher osmotic pressure. Osmotic pressure is commonly expressed in units of pressure, such as pascals (Pa) or atmospheres (atm).
ψs=-π
ψs (solute potential) and π (osmotic pressure) are numerically equal but opposite in sign.
Significance of osmosis
Osmosis is significant in various biological, physiological, and industrial processes. Here are some key significances of osmosis:
- Cellular Function: Osmosis plays a vital role in maintaining proper water balance and hydration of cells. It allows for the movement of water and solutes across cell membranes, ensuring optimal cellular function and stability.
- Nutrient Uptake: Osmosis facilitates the uptake of essential nutrients and minerals by plants and other organisms. Water moves through osmosis, carrying dissolved nutrients from areas of lower concentration to areas of higher concentration, promoting efficient nutrient absorption.
- Turgor Pressure: Osmosis creates turgor pressure in plant cells, providing structural support and helping plants maintain their shape and rigidity. Turgor pressure is crucial for plant growth, cell expansion, and upright positioning.
- Osmoregulation: Osmosis enables organisms to regulate and maintain proper internal osmotic balance. Through osmoregulation, organisms control water uptake or loss to prevent cellular dehydration or excessive swelling.
- Biological Processes: Osmosis is involved in numerous biological processes, including kidney function, blood pressure regulation, nutrient transport in the body, and gas exchange in the respiratory system.
- Industrial Applications: Osmosis finds applications in various industrial processes. Reverse osmosis, for example, is used for water purification and desalination, while osmotic pressure can drive processes like osmotic filtration and separation of substances.
Frequently asked questions on Osmosis
What do you mean by osmosis?
Osmosis is a special type of diffusion which involves movement of water molecules from a region of their higher concentration to a region of their lower concentration through a semi-permeable membrane.
What is the difference between osmosis and diffusion?
Osmosis involves the transfer of solvent particles from a less concentrated solution to a more concentrated one, while diffusion refers to the movement of particles from an area of higher concentration to an area of lower concentration.
What is osmotic pressure?
Osmotic pressure refers to the pressure exerted by a solvent as it moves through a semipermeable membrane to equalize the concentrations of solute on both sides. Osmotic pressure is directly proportional to the solute concentration, meaning that higher solute concentrations result in higher osmotic pressures.
What is plasmolysis?
Plasmolysis is a process that occurs when a plant cell is placed in a hypertonic (higher solute concentration) solution, causing water to move out of the cell. As a result, the cell's protoplast (the living part of the cell) shrinks away from the cell wall.
Describe the factors that influence the rate of osmosis.
The rate of osmosis is influenced by several factors, including the concentration gradient between the solutions, temperature (higher temperature increases the rate), pressure (increased pressure reduces the rate), surface area of the membrane, and the permeability of the membrane to the solute and solvent molecules.







