Table of Contents
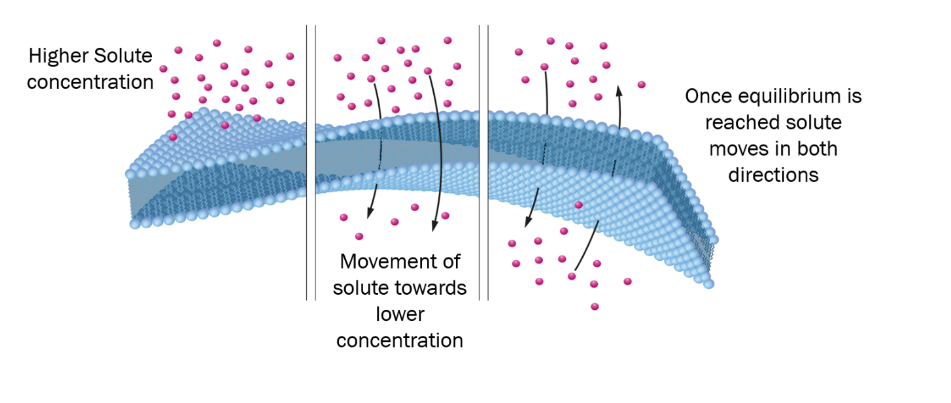
Diffusion
Diffusion is the spontaneous movement of particles, molecules, or ions from an area of higher concentration to an area of lower concentration, resulting in the equalization or distribution of substances.
Brief Overview of Diffusion
Diffusion is a physical process in which particles, molecules, or ions move from an area of higher concentration to an area of lower concentration. It occurs spontaneously and is driven by the random thermal motion of particles. Diffusion is a fundamental mechanism for the mixing, spreading, and transport of substances in various systems, such as gases diffusing in the air, solutes spreading in liquids, or molecules moving across cell membranes. It plays a crucial role in biological, physical, and chemical processes, promoting equilibrium and the distribution of substances.
Types of diffusion
There are several types of diffusion, each characterized by the specific mechanism or factors influencing the movement of particles. Here are some common types of diffusion:
- Simple Diffusion: Simple diffusion refers to the movement of particles directly through a permeable membrane or across a concentration gradient without the involvement of transport proteins. It occurs for small, non-polar molecules or gases, such as oxygen and carbon dioxide.
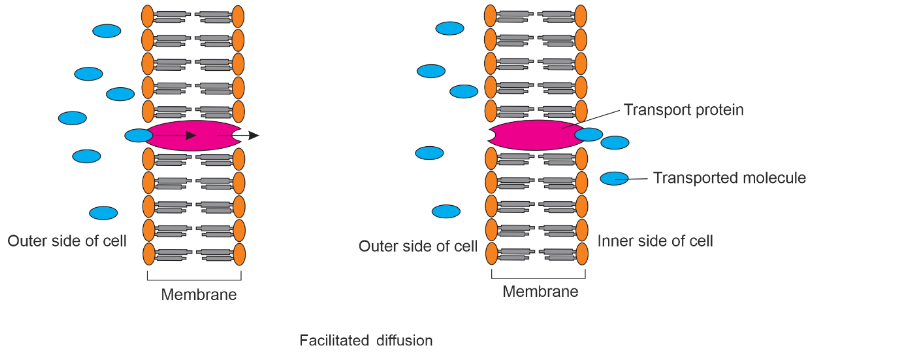
- Facilitated Diffusion: Facilitated diffusion is a type of diffusion that involves the movement of particles across a membrane with the assistance of specific transport proteins. These proteins act as channels or carriers to facilitate the transport of larger or charged molecules, such as glucose or ions, across the membrane.

- Osmosis: Osmosis is a specific type of diffusion that specifically refers to the movement of water molecules across a semi-permeable membrane. It occurs in response to differences in solute concentration and aims to equalize the concentration of solutes on either side of the membrane.
Factors affecting diffusion
Several factors can influence the rate and extent of diffusion. Here are some key factors that affect diffusion:
Concentration Gradient: The concentration gradient, which is the difference in concentration between two regions, is a primary driving force for diffusion. The larger the concentration difference, the faster the rate of diffusion.
Temperature: Temperature affects the kinetic energy of particles. Higher temperatures increase the kinetic energy, leading to faster movement and collisions between particles, thus promoting faster diffusion.
Molecular Size and Mass: Smaller and lighter molecules tend to diffuse more rapidly compared to larger and heavier molecules. This is because smaller molecules have higher average speeds and are more likely to move quickly through spaces between other particles.
Pressure Gradient (for gases): In the case of gases, differences in pressure can affect the rate of diffusion. Gases move from areas of higher pressure to lower pressure, resulting in diffusion.
Examples of diffusion
- Here are few examples of diffusion:
- Perfume spreading in a room.
- Sugar dissolving in coffee
- Oxygen exchange in the lungs
- Food colouring spreading in water.
- Ink spreading in water.
In each case, particles or molecules move from higher concentration to lower concentration, resulting in the even distribution of substances.
Significance of diffusion
Role of diffusion in plants
- Gas exchange: Diffusion allows the exchange of gases, such as carbon dioxide and oxygen, in plant tissues during photosynthesis and respiration.
- Nutrient absorption: Diffusion facilitates the movement of water, minerals, and nutrients from the soil into plant roots and further transport to different plant parts.
- Transpiration: Diffusion enables the movement of water vapor from the leaf surface to the atmosphere, aiding in the transport of water and maintaining plant hydration.
Role of diffusion in animals
- Respiratory gas exchange: Diffusion at alveoli of lungs, facilitates the exchange of oxygen and carbon dioxide in the animals.
- Nutrient absorption: In the digestive system, diffusion enables the movement of nutrients from the intestinal lumen into the bloodstream for distribution to cells and tissues.
- Waste elimination: Diffusion aids in the removal of waste products, such as carbon dioxide and metabolic byproducts, from cells and tissues for excretion.
Frequently Asked Questions on Diffusion
What is diffusion?
Diffusion is the spontaneous movement of particles, molecules, or ions from an area of higher concentration to an area of lower concentration, resulting in the equalization or distribution of substances.
What are various types of diffusion?
The various types of diffusion include: Simple diffusion. Facilitated diffusion. Osmosis.
What is facilitated diffusion?
Facilitated diffusion is a process by which hydrophilic molecules are transported across cell membranes with the help of specific protein channels or carriers. It is a type of passive transport because it does not require the expenditure of energy by the cell.
What are the factors that affect the rate of diffusion?
Factors affecting diffusion include concentration gradient (difference in concentration), temperature (higher temperature increases diffusion rate), surface area (larger area enhances diffusion), and the size and nature of molecules or ions involved.
What is the difference between diffusion and osmosis?
Diffusion is the movement of molecules or ions from an area of higher concentration to an area of lower concentration, while osmosis is a special type of diffusion of water molecules.