Table of Contents
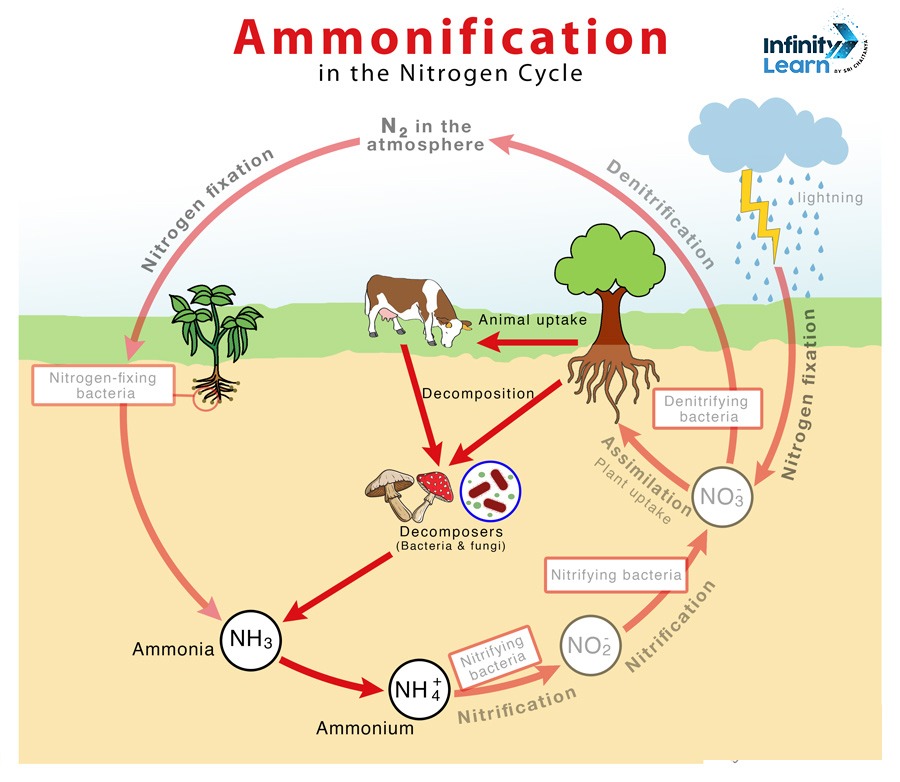
Ammonification Definition: Ammonification is an important step in the nitrogen cycle. It involves the transformation of nitrogen from dead plants and animals into inorganic forms like ammonia (NH₃) or ammonium ions (NH₄⁺) through the actions of decomposers such as bacteria and fungi. This process returns nitrogen to the soil, where it becomes available for plants to absorb and use.
Ammonification in Nitrogen Cycle
Ammonification is an essential part of the nitrogen cycle, which is important for providing living organisms with the nitrogen they need. The following is a brief outline of the process:
- Decomposition: When plants and animals die, decomposers like bacteria and fungi break down their organic matter.
- Conversion: During this breakdown, organic nitrogen compounds (such as proteins and nucleic acids) are converted into ammonia (NH₃) or ammonium ions (NH₄⁺).
- Ammonification: The final step in this process is ammonification, during which inorganic nitrogen compounds are converted into ammonium ions. The ammonia or ammonium ions are then released into the soil, where plants can absorb them.
This process helps recycle nitrogen in the ecosystem, making it available for plants to use for growth. Without ammonification, the nitrogen cycle would be incomplete, and there would be less usable nitrogen for plants and other organisms.
Ammonification Reaction
Ammonification is an important part of the nitrogen cycle where organic nitrogen compounds are changed into ammonia (NH₃) or ammonium ions (NH₄⁺).
- Decomposition of Organic Matter: Decomposers, such as bacteria and fungi, break down organic nitrogen compounds, like proteins and nucleic acids.
- Formation of Ammonia: During this decomposition, the organic nitrogen is converted into ammonia (NH₃).
- Conversion to Ammonium: Ammonia then reacts with water to form ammonium ions (NH₄⁺).
The overall chemical reaction for ammonification can be represented as:
R−NH2+ 2H2O → NH4+ OH –+ROH
In this equation:
- R−NH2 represents an organic nitrogen compound.
- NH4+ is the ammonium ion.
- OH – is the hydroxide ion.
- ROH is the remaining organic molecule.
This process is vital for recycling nitrogen in ecosystems, making it available for plants to absorb and use for growth.
Ammonification Formation
Ammonification Formation is the process through which organic nitrogen compounds are converted into ammonia (NH3). This transformation is carried out by microorganisms, mainly bacteria and fungi, that decompose organic matter such as dead plants, animals, and waste.
During ammonification, the process begins with the decomposition of this organic matter into simpler substances. Following this, proteins are broken down into amino acids through a process called proteolysis. Finally, the amino acids undergo deamination, where they lose their amino groups, resulting in the release of ammonia.
Ammonification Examples
Ammonification is a key part of the nitrogen cycle where decomposers, such as bacteria and fungi, convert organic nitrogen from dead plants and animals into ammonia or ammonium ions. Here are some simple examples:
- Animal Waste: Bacteria break down organic nitrogen compounds in animal waste, like urea, into ammonia.
- Decay of Dead Plants and Animals: When plants and animals die, decomposers turn their proteins and nucleic acids into ammonia.
- Soil Fertilization: Organic fertilizers, such as manure, are broken down by soil microorganisms, releasing ammonia that plants can use.
- Composting: Organic waste like food scraps, leaves, and grass clippings are decomposed by microorganisms into ammonia, enriching compost with nitrogen.
- Aquatic Ecosystems: Dead aquatic plants and animals are decomposed by bacteria in water, converting organic nitrogen into ammonia that aquatic plants and algae can use.
- Sewage Treatment: In wastewater treatment plants, bacteria break down organic nitrogen in sewage into ammonia, which is important for nitrogen removal.
What is Difference Between Ammonification and Nitrification?
Ammonification and nitrification are key processes in the nitrogen cycle, each playing a distinct role in converting nitrogen into forms usable by plants. The table below outlines their differences and functions:
| Difference Between Ammonification and Nitrification | ||
| Aspect | Ammonification | Nitrification |
| Definition | Converts organic nitrogen into ammonia or ammonium ions. | Converts ammonia into nitrites and then into nitrates. |
| Processes |
|
|
| Microorganisms | Bacteria (e.g., Bacillus, Proteus) and fungi (e.g., Clostridium, Pseudomonas) | Bacteria (e.g., Nitrosomonas, Nitrobacter) |
| Examples |
|
|
| Role in Nitrogen Cycle | Converts nitrogen into ammonia, which can be further used or processed. | Converts ammonia into nitrates that plants readily absorb. |
| End Products | Ammonia (NH₃) or Ammonium ions (NH₄⁺) | Nitrates (NO₃⁻) and Nitrites (NO₂⁻) |
| Impact on Plants | Ammonia is supplied for plant use or further processing into nitrates. | Supplies nitrate that are directly absorbed by plants for growth. |
What is Difference Between Ammonification and Denitrification?
Ammonification and denitrification are different steps in the nitrogen cycle, each with its role. The table below provides the difference between Ammonification and Denitrification in the nitrogen cycle.
| Difference Between Ammonification and Denitrification | ||
| Aspect | Ammonification | Denitrification |
| What It Does | Turns organic nitrogen from dead plants and animals into ammonia or ammonium ions. | Turns nitrates and nitrites into nitrogen gas (N₂) or nitrous oxide (N₂O), which goes into the air. |
| Processes |
|
|
| Microorganisms | Bacteria (e.g., Bacillus, Proteus) and fungi (e.g., Clostridium, Pseudomonas) | Bacteria (e.g., Pseudomonas, Paracoccus) |
| Role in the Cycle | Converts nitrogen from dead matter into ammonia, which can be turned into nitrates for plants. | Finishes the nitrogen cycle by sending nitrogen back into the air, reducing soil nitrogen. |
| End Products | Ammonia (NH₃) or Ammonium ions (NH₄⁺) | Nitrogen gas (N₂) or Nitrous oxide (N₂O) |
| Impact on Ecosystem | Adds ammonia that plants can use or convert into nitrates. | Lowers soil nitrogen by turning nitrates into gases that are released into the atmosphere. |
FAQs on Ammonification
What is the ammonification process?
Ammonification is when microorganisms break down organic nitrogen from dead organisms and waste into ammonia (NH₃) or ammonium ions (NH₄⁺). This process turns nitrogen into a form that plants and other organisms can use.
What is also known as ammonification?
Ammonification is also called nitrogen mineralization. It refers to the conversion of organic compounds into inorganic forms like ammonia during decomposition.
Is ammonification a denitrification?
No, ammonification and denitrification are different processes. Ammonification converts organic nitrogen into ammonia, while denitrification reduces nitrates to nitrogen gas (N₂) or nitrous oxide (N₂O) and releases them into the atmosphere.
What is the difference between ammonification and nitrification?
Ammonification turns organic nitrogen into ammonia (NH₃) or ammonium ions (NH₄⁺). Nitrification, on the other hand, converts ammonia into nitrites (NO₂⁻) and then into nitrates (NO₃⁻).
What is an example of ammonification?
An example of ammonification is when soil bacteria and fungi decompose dead plant material, breaking down proteins and nucleic acids to release ammonia into the soil.









