Table of Contents
Dimensions Of Universal Gas Constant: The gas constant R is the constant for each individual ideal gas or ideal gas mixture. M gas R l = p = rRT where, R is the gas constant or universal gas constant. The molar constant of a gas (also called the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol R. It is the molar equivalent of Boltzmann’s constant, which expresses temperature in terms of energy added per mole, i.e. pressure multiplied by volume, rather than energy raising the temperature of each particle. The universal gas constant R is a number that satisfies proportionality.
“We also define a new gas constant, R, which is equal to the universal gas constant divided by the mass per mole of gas”
Knowing these values, R is calculated using the equation PV = nRT. Therefore, we will say that the constant k is equal to the value for all gases. In addition, the constant is the same for all gases, provided that the mass of the gas being compared is a mole or molecular weight in grams. Let us learn more about the Dimensions Of Universal Gas Constant.
Avogadros’ Law:
Avogadros’ Law states that one mole of any gas contains the same number of molecules, equal to 6.02214 x 1023. In fact, by definition, if the thermodynamic state of a gas satisfies Clapeyron’s equation p = R^(13) where Ris is the specific gas constant, defined as the ratio of the universal gas constant to the molar mass of the gas (or equivalently, Bohr the ratio between the Zeman constant and the average mass of the gas molecules). In general, an ideal gas will remain constant at any pressure, temperature or volume. Molar, Universal, Ideal Gas Constant, si Units, Imperial Units, Formula, Numerical, Specific Gas Constant, 8.314 J/mol/k, 0.082 l atm/mol/k 7 Boltzmann Constant and Ideal Gas Constant.
Gas properties:
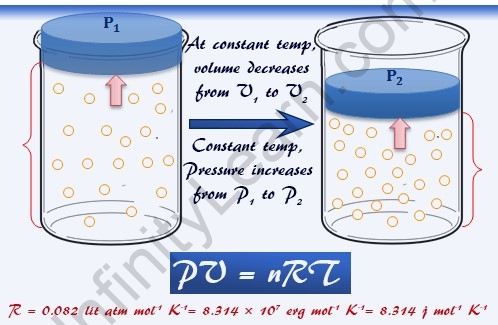
Like pressure, volume and temperature have long been measured in different and independent units. The experimental value of Boyle’s law constant is determined by the product of pressure P and volume V at a constant temperature T and a fixed air mass m. The units used to express pressure, volume and temperature will determine the correct form of the gas constant as required by dimensional analysis, the most common values being 0.08206 l atm mol-1 K-1 and 8.314 kPa l mol- one. K – 1. Dimensional formula for temperature and volume = \[M^{0}L^{0}T^{0}K^{1} and M^{0}L^{3}T^{0} \] .
Both the Avogadros number and the Boltzmann constant have received exact numerical values. However, USSA1976 acknowledges that this value is inconsistent with the reported values for the Avogadro constant and the Boltzmann constant.
The two digits in parentheses represent the uncertainty (standard deviation) of the last two digits of the value. Citations is the number of other articles that cite this article, counted by Crossref and updated daily.
French chemist Henri Regnault is best known for his work on measuring the thermal properties of gases. A gas or mixture of gases or mixtures of gases (gas R or simply R) whose specific gas constant or individual gas constant is divided by the Boltzmann constant by the molar mass
Formula:
Dimensions Of Universal Gas Constant: PV = nRT, where n is the number of moles, or PV = mRT, where m is the mass. It can be expressed in any set of units representing work or energy (such as joules), units representing degrees of temperature on an absolute scale (such as Kelvin or Rankine), and any system of units representing the mole or similar pure number, which allows the macroscopic mass equation and fundamental numbers of particles in a system such as an ideal gas (see Avogadro’s constant). It is a physical constant that is present in many fundamental equations of the physical sciences, such as the ideal gas law, the Arrhenius equation, and the Nernst equation. If we look at speed, we can see that it has the dimension of length divided by the dimension of time and acceleration.
Time, of course, has a temporal dimension that is greater than the dimension of length. Now we can see that the dimension of time here will shrink with one of these two, leaving us with only duration in time. The lengths are combined into one, and so we have the length squared over time, which is where we started, and so the D part is also a measurement. When one of these three changes for a given mass of gas, at least one of the other two changes so that the pV/T expression remains constant.
Gas Constant:
The gas constant is equivalent to the Boltzmann constant expressed only in units of energy per temperature per mole, while the Boltzmann constant is expressed in units of energy per temperature per particle. While the Boltzmann constant is the same for all ideal gases, a particular (or individual) gas constant applies to a particular gas (or mixture of gases, such as air). Chemical and physical equations usually include “R”, which is the symbol for the gas constant, molar gas constant, ideal gas constant, or universal gas constant. The gas constant (also known as the molar, universal, or ideal gas constant)
is a physical constant found in numerous fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation.
The Nernst equation is an important equation used in chemical kinetics. The Nernst equation is an electrochemical equation that relates the potential of an electrochemical reaction to the potential of a standard electrode. The isothermal change corresponds to k = 1 and Equation 6.27 becomes (6.31) PV = constant for an ideal gas. As the name suggests, isothermal change occurs at a constant temperature.
In the general case, it will remain unchanged for an ideal gas at any pressure, temperature, or volume. Gases have various properties that we can observe with the senses, including gas pressure p, temperature T, mass m, and volume V containing the gas. An ideal or perfect gas obeys the equation (6.20) P V = R T RMM RMM, where R is the universal gas constant, T is the absolute temperature, and RMM is the relative molecular weight conversion factor of the gas. Simple gas equation Before considering the ideal gas equation, let’s formulate four gas variables and a constant for better understanding.
Get the most Important Questions in Physics, Chemistry, Math, and Biology.
Make your IIT Dream come true with Infinity Learn.
FAQs:
What is dimensional formula of R?
R= 0.0821 litre atm/ k mol.
What is the dimension of a real gas equation?
a=ML5T−2
Is the gas constant always the same?
Yes
Infinity Learn App
Now you can find answers to all your subject queries & prepare for your Exams on our Ultimate Learning App for CBSE and K-12 – Infinity Learn.



