Table of Contents
Band theory is derived from the theory of molecular orbitals and is based on quantum mechanics. When several atoms are combined to form a molecule, their atomic orbitals combine to form a layer of molecular orbitals, each with a different energy. The energy levels in the giant molecule are so close that they can be considered a continuum. Even though the different atomic orbitals interact with one another, they do not mix. They instead form a layer. Because there is a difference in energy in the same orbital of another atom, the same atomic orbital “n” combines to form “n” molecular orbit, forming a layer. The continuous band refers to these continuous layers of energy levels. The difference in energy levels is referred to as the energy gap or bandgap. The energy bands are close together, and the two uppermost bands are the valence band and the conduction band.
Overview of Band Theory of Conductors, Semiconductors and Insulators
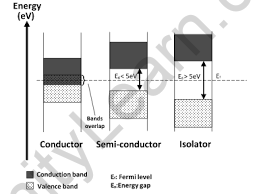
Plotting the available energies for electrons in the materials is a useful way to visualise the difference between conductors, insulators, and semiconductors. The available energy states form bands rather than discrete energies as in the case of free atoms. The presence or absence of electrons in the conduction band is critical to the conduction process. In insulators, electrons in the valence band are separated from the conduction band by a large gap; in conductors such as metals, the valence band overlaps the conduction band; and in semiconductors, the gap between the valence and conduction bands is small enough that thermal or other excitations can bridge the gap. With that kind of a small gap, the presence of a small percentage of a doping material can dramatically increase conductivity.
The electrons in the valence band have lower energy levels and are referred to as valence electrons. The innermost electrons in an atom are much less attracted by neighbouring atoms and have discrete energy levels. We can say that the conduction band is empty in a semiconductor. If enough energy is applied, electrons in the valence band will jump to the conduction band because the energy gap between the valence and conduction bands in a semiconductor is smaller. Doping can enhance the phenomenon of electrons jumping to the conduction band. Doping is the addition of impurities such as intrinsic material at the junction or gap between the conduction and valence bands. It closes the gap by allowing holes and electrons to pass through.
Band Theory of Solids
The band theory of solids is a conceptual model that explains the states of electrons in solid materials that can only have energy values within certain ranges. The quantum state that an electron takes inside a metal solid is described by the band theory of solids. Every molecule contains a number of discrete energy levels. The behaviour of electrons within a molecule is well explained by band theory. Band Theory arose from scientific knowledge gained during the quantum revolution.
Following Pauli’s exclusion principle, electrons in atoms are filled in their respective energy orbits. A molecular orbit is formed when two atomic orbitals combine to form a molecular orbit with two distinct energy levels. 1023 stacked up lines confined in a tiny space would resemble a band in solids. As a result, an energy continuum known as energy bands is formed. By plotting available energies for an electron in a material, band theory helps to visualise the difference between a conductor, a semiconductor, and an insulator.
There are many energy bands in solid band theory, but the three most important energy bands in solids are as follows:
- Valence Band: The valence band is the energy band that consists of valence electron energy levels. The valence band exists beneath the conduction band, and the electrons in this band are loosely bound to the atom’s nucleus.
- Conduction Band: The conduction band is the energy band that consists of free electron energy levels. External energy must be applied in order for valence electrons to be pushed into the conduction band and become free.
- Forbidden Band: The forbidden band, also known as the forbidden gap, is the energy gap between the valence band and the conduction band. The electrical conductivity of a solid is determined by the forbidden gap as well as the materials’ classification as conductors, semiconductors, or insulators.
If you only have one atom or if you have gas. Atoms in gas are far apart/infinitely far apart; we can treat them as single atoms. Every atom in this system has a discrete energy level; if an electron wants to move from one level to another, it must jump because no continuous energy is available (It is similar to steps).
As atoms come closer together and eventually form a solid, they form an energy continuum, which we call a band. The available energy levels are constant within the bands. As a result, it comes as no surprise that the name of this theory is “The band theory of solids.”
Band Theory of Conductors, Semiconductors and Insulators
Here is explanation of conductor, semiconductor and insulator on the basis of band theory:
There are no band gaps between the valence and conduction bands in a conductor. The conduction and valence bands partially overlap in some metals. This means that electrons can freely move between the valence and conduction bands.
Only a portion of the conduction band is filled. This means that there are places for electrons to move. Electrons from the valence band are free to move when they enter the conduction band. This enables conduction.
The gap between the valence band and the conduction band is smaller in a semiconductor. There is enough energy available at room temperature to move some electrons from the valence band into the conduction band. This allows for some conduction to occur.
A semiconductor’s conductivity increases as temperature rises because more electrons have enough energy to move into the conduction band.
The valence band and conduction band of an insulator are separated by a large gap. Because no electrons can move up to the conduction band, the valence band is full. As a result, the conduction band is completely empty.
Only electrons in a conduction band can move easily, so because an insulator’s conduction band lacks electrons, the material cannot conduct.
Band Theory of Conductors
The difference in conductivity is also affected by temperature. When the temperature rises, the atoms in the metal move even faster, causing electrons to be forced or strained in their motions or movements. As a result, resistance grows. (Au) Gold and (Ag) Silver are the best conductors of electricity, but they are used infrequently due to their high cost (like gold). As a result, the alternative components used in microchips in semiconductors are (Al) Aluminium and (Cu) Copper.
Metals like copper (Cu) and aluminium (Al) have no forbidden gap resentment between their conduction and valence bands.
The valence band and conduction band overlap here. As a result, even at room temperature, a large number of electrons are available for conduction.
As a result of the use of any extra or additional energy, these metal types have a large number of free electrons and are thus known as good conductors.
In such a conductor, either the valence band is not completely filled with electrons or the “filled valence band” overlaps with the vacant conduction band.
In general, both states occur concurrently, so electrons can move within the partially filled (V.B) valence band or within the bands that overlap.
In a conductor, there is no band gap between the conduction and valence bands.
In the scenario of a conductor, the band occupied by the final energy levels is only partially filled. According to “Pauli’s exclusion principle,” the lowest levels are occupied one at a time by the possible electrons.
This leaves an unoccupied portion of the band known as the conduction band.
Electrons move freely in the conduction band, which is partially filled in the valence band.
At absolute zero temperature, electrons occupy the topmost energy level in the partially filled conduction band, which is referred to as the “Fermi level,” and the equivalent energy is referred to as the “Fermi energy.”
Band Theory of Semiconductors
The band theory of semiconductors explains how their electrical properties vary with temperature. According to this theory, semiconductors behave as insulators at absolute zero. At this point, the valence band is completely filled, and the conduction band is empty, leaving no room for electron movement, which prevents electrical conduction.
As the temperature rises above absolute zero but remains below the material’s melting point, semiconductors begin to conduct electricity. This happens due to the small band gap—typically around 1 electron volt (eV)—between the valence and conduction bands. With sufficient thermal energy, some electrons gain enough energy to jump from the valence band to the conduction band. This movement of electrons creates charge carriers that enable electrical conduction.
In essence, while semiconductors act as insulators at very low temperatures, their conductivity increases with temperature as electrons move across the energy gap between the valence and conduction bands, allowing for electrical conduction to occur.
Band Theory of Insulators
In insulators, the gap between the conduction band and the valence band is very large. This gap, called the “forbidden energy gap,” prevents electrons from moving freely, which is why insulators cannot conduct electricity.
The energy gap in insulators is usually around 7 electron volts (eV), meaning it takes a lot of energy for an electron to jump from the valence band to the conduction band. For example, diamond is an insulator with a gap of about 6 eV. This large gap makes it difficult for electrons to move, so insulators can only conduct electricity under extremely high temperatures or strong voltages.
In rare cases, an insulator can break down and start conducting electricity. This is known as “insulation breakdown.” Common insulating materials include wood, glass, paper, and mica.
Energy Bands Terms – Valence Band, Forbidden Band, and Conduction Band
Valence Band
The valence band is the range of energy in a solid where the outermost (valence) electrons of an atom are found. At absolute zero temperature, this is the highest energy range that is completely filled with electrons. These electrons in the valence band are responsible for bonding between atoms. However, they have lower energy compared to the electrons in the conduction band. The ability of a material to conduct electricity depends on whether electrons can move from the valence band to the conduction band. If the electrons can easily make this jump, the material can conduct electricity.
Forbidden Band (Energy Gap):
The forbidden band, also known as the forbidden energy gap, is the range of energy between the valence band and the conduction band where no electrons can exist. In this region, no electron energy levels are allowed, meaning that electrons cannot occupy this energy range. The size of the forbidden band determines the electrical conductivity of a material. For example, a large forbidden band makes a material an insulator because electrons cannot easily jump from the valence band to the conduction band. The Fermi energy level is often associated with this gap and represents the highest energy electrons at absolute zero temperature.
Conduction Band
The conduction band is the range of energy levels where free electrons exist. These electrons have enough energy to move freely and conduct electricity. In a solid, electrons in the valence band can be excited (with enough energy) to jump into the conduction band, where they are no longer bound to a specific atom and can move through the material. The conduction band is either empty or partially filled, depending on the material. Electrons in this band have higher energy than those in the valence band, allowing them to contribute to electrical conductivity when an external force, like an electric field, is applied. The conduction band is positioned above the Fermi level and represents the lowest level where electrons can move freely.
FAQs on Band Theory of Conductors, Semiconductors and Insulators
What is the band theory of solids?
The band theory of solids is a conceptual model that describes the states of electrons in solid materials that can only have energy values within certain ranges.
How do you explain solid categorization on the basis of band theory?
Solids can be classified as conductors, insulators, or semiconductors based on the distribution of electron energies in each atom. In a semiconductor or insulator, however, there is a gap between the bottom of the conduction band and the top of the valence band.
What do you mean by a forbidden gap as used in the band theory?
The forbidden energy gap that exists between the valence and conduct bands. It is considered as the distance between the valence and conduction bands. If this gap is larger, it indicates that valence band electrons are tightly bound to the nucleus.
What is the band theory of a semiconductor?
The band theory of a semiconductor explains how its electrical properties depend on the energy gap between the valence band and the conduction band. In a semiconductor, this energy gap is relatively small, allowing some electrons to gain enough energy to move from the valence band to the conduction band at room temperature. Once in the conduction band, these electrons are free to move and conduct electricity. The ability to conduct electricity increases as the temperature rises, as more electrons gain sufficient energy to make the jump to the conduction band. This characteristic makes semiconductors essential for electronic devices, as their conductivity can be controlled by external factors such as temperature or doping.
What is a conductor using the band theory?
Using the band theory, a conductor is a material where the valence band and the conduction band either overlap or are very close to each other, allowing electrons to move freely between them. This means there is no significant energy gap that restricts electron movement. As a result, electrons in the valence band can easily move to the conduction band and contribute to electrical conductivity. Since these electrons are free to move throughout the material, conductors, like metals, can efficiently carry an electric current when an external voltage is applied.
What is the energy band theory of conductors, semiconductors, and insulators?
The energy band theory of conductors, semiconductors, and insulators explains the differences in electrical conductivity based on the energy gap between the valence band and the conduction band. In conductors, the valence and conduction bands overlap, allowing electrons to move freely, resulting in high electrical conductivity. In semiconductors, there is a small energy gap between the two bands, and only a few electrons can move to the conduction band, allowing limited conductivity that can be increased with temperature or external energy. In insulators, the energy gap is large, making it difficult for electrons to move from the valence band to the conduction band, which prevents electrical conduction. This band structure defines the material's behavior as a conductor, semiconductor, or insulator.








