JEE Main Previous Year Papers Questions With Solutions Chemistry Chemical Bonding and Molecular Structure

Ans.(1) In NH3 and BF4– the hybridisation is sp3 and the bond angle is almost 109°28 ’
2.In XeF2, XeF4, XeF6 the number of lone pairs of Xe are respectively (2002)
(1)2, 3, 1 2) 1, 2, 3 3) 4, 1, 2 4) 3, 2, 1
Ans.(4) XeF2 sp3d 4 lone pairs
XeF4 sp3d2 2 lone pairs
XeF6 sp3d3 1 lone pair

Ans.

4.Which of the following statements is true?
(1)HF is less polar than HBr (2002)
(2)absolutely pure water does not contain any ions
(3)chemical bond formation take place when forces of attraction overcome the forces of repulsion
(4)in covalency transference of electron takes place
Ans.(3) Bond formation is exothermic.
5.In which of the following species is the underlined carbon having sp3 hybridisation ?(2002)
1) CH3COOH 2) ch3ch2oh
3) GH3COCH3 4) CH2 = CH – CH3
Ans.(2) In molecules (a), (c) and (d), the carbon atom has a multiple bond, only in molecule (b) carbon has sp3 hybridisation.
6.In the anion HCOO” the two carbon -oxygen bonds are found to be of equal length. What is the reason for it ? (2003)
(1)The C=0 bond is weaker than the C-O bond
(2)The anion HCOO” has two resonating structures
(3)The anion is obtained by removal of a proton from the acid molecule
(4)Electronic orbitals of carbon atom are hybridised
Ans.
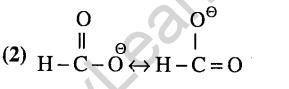

Ans.(2) In H2S, due to low electronegativity of sulphur the L.P.-L.P repulsion is more than B.P.- B.P. repulsion and hence the bond angle in H2S is 92°.
8.The pair of species having identical shapes for molecules of both species is (2003)
1) XeF2, CO 2) BF3, PC13
3) PF5, IF5 4) CF4, SF4
Ans.(1) Both XeF2 and C02 have a linear structure.
9.Which one of the following pairs of molecules will have permanent dipolemoments for both members ? (2003)
1) N02 and C02 2) N02 and 03
3) SiF4 and C02 4) SiF4 and N02
Ans.(2) Both N02 and 03 have angular shape and hence will have net dipole moment.
10.The correct order of bond angles (smallest first) in H2S, NH3, BF3 and SiH4 is (2004)
(1)H2S < SiH4 < NH3 < BF3
(2)H2S < NH3 < BF3 < SiH4
(3)H2S < NH3 < SiH4 < BF3
(4)NH3 < H2S < SiH4 < BF3
Ans.(3)H2S—— > sp3 NH3——– > sp3
BF3—- > sp2 SiH4——– > sp3
11.The bond order in NO is 2.5 while that in NO+is 3. Which of the following statements is true for these two species? (2004)
(1)Bond length in NO+ is greater than in NO
(2)Bond length is unpredictable
(3)Bond length in NO+ in equal to that in NO
(4)Bond length in NO is greater than in NO+
Ans.

12.The states of hybridization of boron and oxygen atoms in boric acid (H3B03) are respectively (2004)
1) sp2 and sp2 2) sp3 and sp3
3) sp3 and sp2 4) sp2 and sp3
Ans.(4) B is sp2 and O is sp3
13.The maximum number of 90° angles between bond pair of electrons is observed in (2004)
1) dsp3 hybridization 2) sp3d2 hybridization 3) dsp2 hybridization 4) sp3d hybridization
Ans.(2) sp3d2 hybridisation confirms to octahedral or square bipyramidal configuration.All the bond angles are 90° in the structure
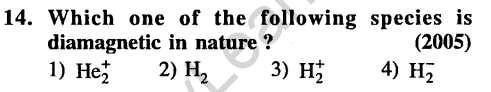
Ans.

15.Lattice energy of an ionic compounds depends upon (2005)
1) Chaige on the ion only
2) Size of the ion only
(3)Packing of ions only
(4)Charge on the ion and size of the ion
Ans.(4) Lattice energy will be more if, charge is high and size is low.
16.The molecular shapes of SF4, CF4 and XeF4 are (2005)
(1)the same with 2, 0 and 1 lone pairs of electrons on the central atom, respectively
(2)the same with 1,1 and 1 lone pair of electrons on the central atoms, respectively
(3)different with 0, 1 and 2 lone pair of electrons on the central atoms, respectively
(4)different with 1, 0 and 2 lone pairs of electron on the central atoms respectively
Ans.(4) Number of lone pairs on S is 1 and on Xe is 2
17.The number and type of bonds between two carbon atoms in calcium carbide are (2005)
1) One sigma, one pi 2) One sigma, two pi
3) Two sigma, one pi 4) Two sigma, two pi
Ans.
![]()
18.
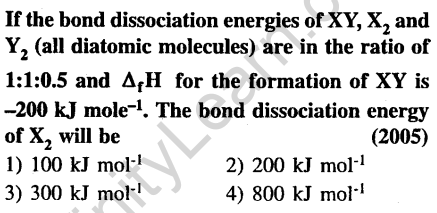
Ans.
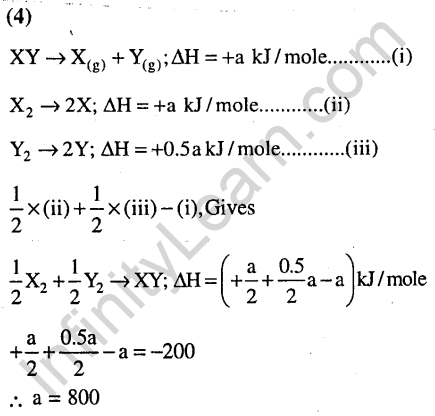

Ans.

20.Among the following mixtures, dipole-dipole as the major interaction, is present in (2006)
(1)benzene and ethanol
(2)acetonitrile and acetone
(3)KC1 and water
(4)benzene and carbon tetrachloride
Ans.(2) CH2 = CHCN and CH3COCH3 are polar molecules.
21.In which of the following molecules/ions are all the bonds not equal ? (2006)
1) SF4 2) SiF4 3) XeF4 4) BF4
Ans.(1) S in SF4 has one Lone pair
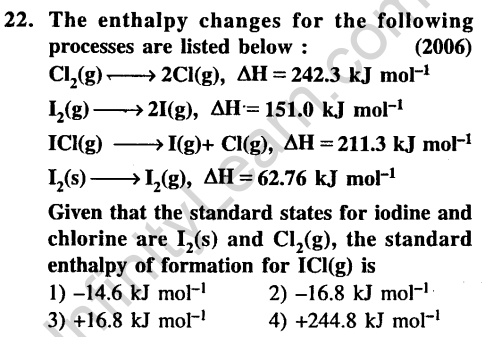
Ans.
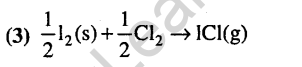

Ans.
24.Which of the following hydrogen bonds is the strongest ? (2007)
1) O-H………… N 2) F-H…………. F
3) O-H………… O 4) O-H………… F
Ans.(2) The hydrogen bond in HF is strongest, because fluorine is the most electronegative element.
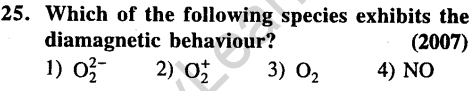
Ans.


Ans.(1) Both are isoelectronic and have same bond order.

Ans.
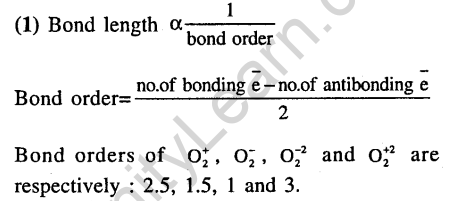
28. The bond dissociation energy of B – F in BF3 is 646 kj mol’1 where as that of C – F in CF4 is 515 kj mol-1. The correct reason for higher B-F bond dissociation energy as compared to that of C – F is (2009)
(1)smaller size of B-atom as compared to that of C -atom
(2)stronger o bond between B and F is BF3 as compared to that between C and F in CF4
(3)significant pn-pn interaction between B and F in BF3 where as there is no possibility of such interaction between C and F in CF..
(4)lower degree of pn-pn interaction between B and F in BF3 than that between C and F in cf4.
Ans.

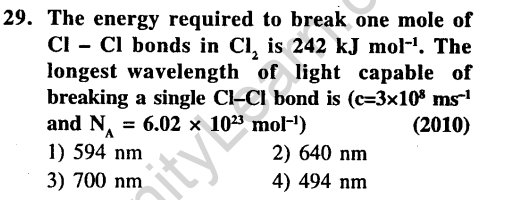
Ans.
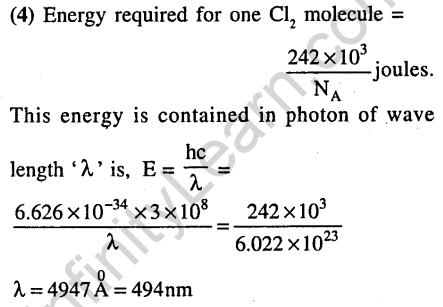
30.Among the following the maximum covalent character is shown by the compound (2011)
(1)A/C/3 2) MgC/2 3) FeC/2 4) SnC/2
Ans.(1) According to Fajan’s rule, polarisation is proportional to charge on cation in turn polarisation is proportional to covalent character A/+3 C/3, Mg+2C/2, Fe+2C/2 and Sn+2 C/2 A/CZ3 is most covalent
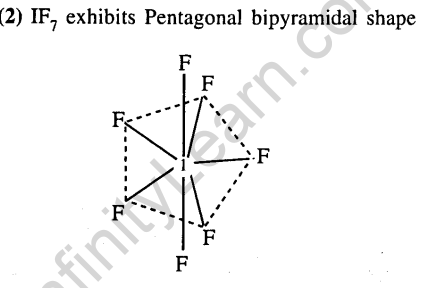
31.The structure of IF7 is (2011)
(1) octahedral
(2)pentagonal bipyramid
(3)square pyramid (4) trigonal bipyramid
Ans.

Ans.
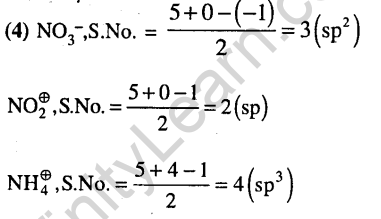
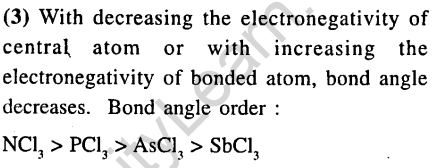
33.The molecule having smallest bond angle is
1) nci3 2)AsCl
3) SbCl3 4) PC13
Ans.
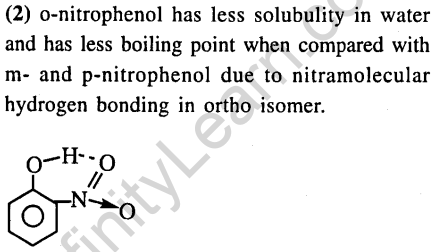
34.Ortho-Nitrophenol is less soluble in water than p-and m-Nitrophenols because (2012)
(1)o-Nitrophenol is more voltaile is steam than those of m- and p- isomers
(2)o-Nitrophenol shows Intramolecular H-bonding
(3)o-Nitrophenol shows Intermolecular H-bonding
(4)Melting point of o-Nitrophenol lower than those of m- and p-isomers
Ans.

Ans.
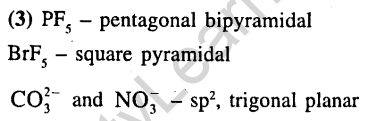
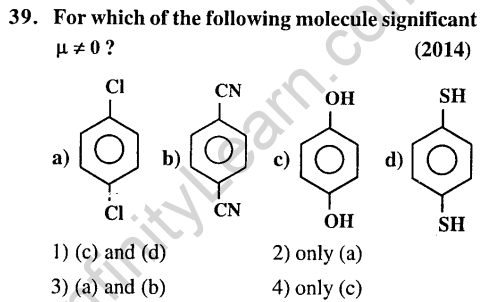
36.Which one of the following molecules is expected to exhibit diamagnetic behaviour ?(2013)
1) C2 2) N2 3) 02 4) S2
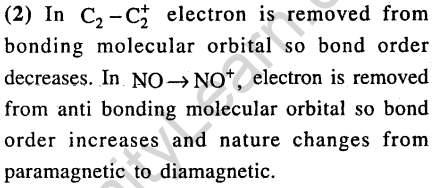
Ans.(1, 2) According to MO theory in N2 and C2 all electrons are paired. So diamagnetic.

Ans.

Ans.

Ans.
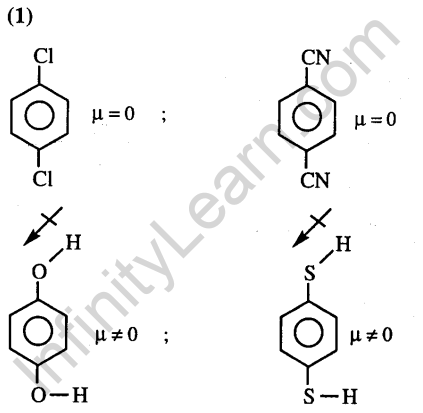
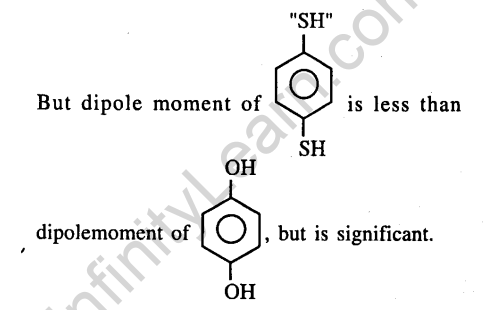
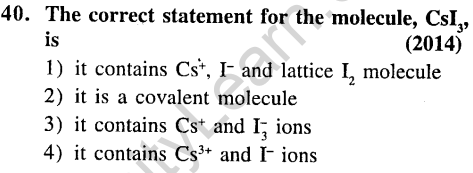
Ans.
![]()
41.The inter molecular interaction that is dependent on the inverse cube of distance between the molecular is (2015)
(1)ion-ion interaction
(2)ion-dipole interaction
(3)London force 4) hydrogen bond
Ans.(4) Hydrogen bond is the type of dipole-dipole interaction.