Table of Contents
Hydrogen bonds are classified into two types: intermolecular hydrogen bonds, which occur between separate molecules, and intramolecular hydrogen bonds, which occur within a single molecule.
The unique physical and chemical properties of compounds containing nitrogen, oxygen, and fluorine are largely attributed to hydrogen bonding. For instance, water’s high boiling point of 100°C, compared to other hydrides with weaker hydrogen bonds, is primarily due to its strong intermolecular hydrogen bonding.
What is Intermolecular Hydrogen Bonding
Intermolecular hydrogen bonding occurs between separate molecules when hydrogen atoms bonded to electronegative atoms interact with nearby electronegative atoms. This type of bonding can occur among molecules of the same substance, such as NH3 or H2O, or between different substances like NH3 and H2O. Essentially, when a hydrogen atom, attached to an electronegative atom, comes close to another electronegative atom, it forms intermolecular hydrogen bonds.
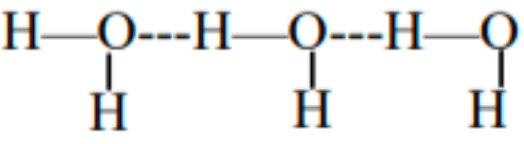
To further explore this, let’s examine the different molecular forces in polymers, which will provide a clearer understanding of the types and branches of intermolecular hydrogen bonding.
Also Check: Types of Intermolecular Forces
Molecular Forces in Polymers
Atoms in molecules are connected by different types of bonds based on their valence electrons. In contrast, molecules interact with each other through weaker bonds, which arise from their electron configurations. Generally, there are two main types of bonding:
- Primary Bonds: Intramolecular forces that hold atoms within a molecule together.
- Secondary Bonds: Intermolecular forces that attract molecules to each other.
Secondary Bonds/Intermolecular Hydrogen Bonding
Secondary bonding refers to the attraction between molecules, often known as intermolecular hydrogen bonding. Unlike primary bonds, which involve atom-to-atom attractive forces, secondary bonds are weaker as they result from intermolecular forces where electrons are neither transferred nor shared. There are three main types of secondary bonding: dipole forces, London dispersion forces, and hydrogen bonding.
Dipole Force
Dipole forces occur in molecules with equal but opposite electrical charges, creating dipoles. For instance, hydrogen chloride molecules form dipoles, where the positive and negative ends attract each other. These interactions result in net intermolecular bonding within the substance.
London Forces
London forces, or dispersion forces, act between nonpolar molecules. Unlike dipole forces, these molecules do not have permanent dipoles. Instead, temporary dipoles arise due to the fluctuating electron distribution within the molecules, leading to temporary attractions.
Hydrogen Bonding
Hydrogen bonding occurs when hydrogen atoms, covalently bonded to a more electronegative atom like oxygen (as in H₂O), interact with electrons in neighboring molecules. This type of bonding is stronger than dipole or London forces and is crucial in forming many polymers.
Difference Between Intermolecular And Intramolecular Hydrogen Bonding
| Aspect | Intermolecular Hydrogen Bonding | Intramolecular Hydrogen Bonding |
| Definition | Bonding between hydrogen atoms of different molecules. | Bonding within a single molecule between different parts. |
| Example | Hydrogen bonding in water (H₂O) between different water molecules. | Hydrogen bonding within a single molecule like salicylic acid. |
| Occurrence | Occurs between separate molecules. | Occurs within the same molecule. |
| Impact on Properties | Influences the bulk properties such as boiling and melting points. | Affects the molecular shape and internal structure. |
| Role in Substance | Determines solubility, viscosity, and surface tension. | Stabilizes the molecule’s structure and affects reactivity. |
| Strength | Generally weaker compared to intramolecular bonds. | Typically stronger due to proximity within the molecule. |
| Structural Influence | Affects the arrangement and interaction of molecules in a substance. | Determines the 3D shape and stability of the individual molecule. |
FAQs on Intermolecular Hydrogen Bonding
What is intermolecular hydrogen bonding?
Intermolecular hydrogen bonding occurs between hydrogen atoms of different molecules, influencing their interaction and properties.
What is an example of an intramolecular hydrogen bond?
An example is the hydrogen bond within a molecule of salicylic acid, where hydrogen bonds occur between different parts of the same molecule.
What is called intermolecular?
Intermolecular refers to interactions or bonds between separate molecules.
What are intermolecular bonds called?
Intermolecular bonds are known as van der Waals forces, including hydrogen bonding, dipole-dipole interactions, and London dispersion forces.
What are the two types of hydrogen bonding?
The two types are intermolecular hydrogen bonding (between molecules) and intramolecular hydrogen bonding (within the same molecule).
Which is stronger, intermolecular or intramolecular hydrogen bonding?
Intramolecular hydrogen bonding is generally stronger than intermolecular hydrogen bonding due to the proximity of interacting sites within the molecule.








