Table of Contents
Class 9 Science Chapter 1: This chapter has been withdrawn from the Revised Term-wise CBSE Syllabus 2025-26.
From the perspective of your CBSE Class 9 Science examination, NCERT Solutions for Class 9 Science (Chemistry) Chapter 1 Matter in Our Surroundings is important study material. The detailed NCERT Solutions for Class 9 Chemistry to all of the exercise questions offered here will aid in your understanding of the chapter’s core ideas.
The matter is a fundamental idea in science, and it serves as the foundation for subjects covered in the following classes. NCERT Solutions for Class 9 Science (Chemistry) Chapter 1 – Matter in Our Surroundings might help you learn more. Solutions are created with the assistance of qualified teachers with extensive conceptual knowledge and years of expertise. The content is well-structured to make learning and understanding easy for pupils. In addition, the NCERT Solutions have been revised to reflect the most recent NCERT syllabus.
We also make certain that the NCERT Solutions for Class 9 Science we supply are tailored to suit a variety of evaluator criteria. This ensures that the responses are extremely relevant and maintain their informational value across courses.
Everything you see around you – from the air you breathe to the food you eat, the clothes you wear, and even the stars in the night sky – is made up of matter. Understanding matter is fundamental to comprehending the world we live in. In this comprehensive guide, we’ll delve into the fascinating world of matter, exploring its definition, nature, different states, properties, and how it changes from one form to another. This article is designed to be your ultimate resource for mastering the concepts of matter in our surroundings, making it ideal for students preparing for exams and anyone curious about the building blocks of the universe.
What is Matter?
Matter Definition
At its core, matter is anything that occupies space and has mass. This simple definition encompasses everything from the smallest atom to the largest galaxy. The physical nature of matter is particulate, meaning it’s composed of tiny, discrete particles rather than being continuous. Imagine a drop of water; it might seem continuous, but under a powerful microscope, you’d see countless individual water molecules.
Characteristics of Particles of Matter
The particles that make up matter exhibit several key characteristics:
- They are continuously moving: Even in what appears to be a stationary object, the particles are constantly vibrating, rotating, and translating. This inherent motion is why gases diffuse quickly and liquids flow.
- They have space between them: The amount of space varies significantly between different states of matter. Solids have very little space, while gases have vast amounts.
- They attract each other: Particles of matter are held together by forces of attraction. The strength of these forces determines many of matter’s properties, such as its rigidity or fluidity.
States of Matter
Matter exists in different forms, commonly known as states. These states are primarily determined by the arrangement and energy of their constituent particles.
Solid, Liquid, Gas
- Solids: Have a definite shape and a definite volume. Their particles are tightly packed and vibrate in fixed positions. Examples include ice, wood, and rock.
- Liquids: Have a definite volume but no definite shape. They take the shape of their container. Their particles are less tightly packed than solids and can slide past each other. Examples include water, oil, and milk.
- Gases: Have neither a definite shape nor a definite volume. They completely fill the container they are in. Their particles are far apart and move randomly and rapidly. Examples include air, oxygen, and nitrogen.
Plasma and Bose-Einstein Condensate (Optional for completeness)
While solids, liquids, and gases are the most common states of matter on Earth, two other states are worth mentioning for their unique properties:
- Plasma: Often referred to as the “fourth state of matter,” plasma is an ionized gas where atoms have lost or gained electrons, resulting in a mixture of ions and free electrons. It’s the most abundant state of matter in the universe, found in stars, lightning, and neon signs.
- Bose-Einstein Condensate (BEC): This state of matter occurs at extremely low temperatures, near absolute zero. In a BEC, a group of bosons is cooled to such a low temperature that their individual identities become indistinguishable, behaving as a single quantum entity. This exotic state has potential applications in quantum computing and precision measurements.
NCERT Solutions Class 9 Science Chapter 1 PDF Download
NCERT Solutions for Class 9 All Subjects
- NCERT Solutions for Class 9 Maths
- NCERT Solutions for Class 9 Science
- NCERT Solutions for Class 9 Social Science
- NCERT Solutions for Class 9 English
Properties and Characteristics of Matter
Beyond its states, matter possesses several measurable properties that help us understand and classify it.
Volume, Mass, Density
- Volume: The amount of space an object occupies. It is typically measured in cubic meters (m3) or liters (L).
- Mass: The amount of matter contained in an object. It is a fundamental property and is typically measured in kilograms (kg) or grams (g). Unlike weight, mass does not change with gravity.
- Density: A measure of how much mass is contained in a given volume. It is calculated as mass divided by volume (rho=fracmV). Density is a crucial property for understanding buoyancy and material properties.
Compressibility & Diffusion
- Compressibility: The ability of a substance to be squeezed into a smaller volume. Gases are highly compressible due to the large spaces between their particles, while liquids are very slightly compressible, and solids are practically incompressible.
- Diffusion: The intermixing of particles of two different types of matter on their own. This phenomenon is a direct consequence of the continuous motion of particles. For example, the smell of an incense stick spreads throughout a room due to diffusion.
Interconversion of States of Matter
Matter can change from one state to another by altering temperature and pressure. These changes are physical changes, meaning the chemical composition of the matter remains the same.
Melting, Freezing, Condensation, Boiling, Sublimation
- Melting (Fusion): The process by which a solid changes into a liquid upon heating (e.g., ice melting into water).
- Freezing (Solidification): The process by which a liquid changes into a solid upon cooling (e.g., water freezing into ice).
- Boiling (Vaporization): The process by which a liquid rapidly changes into a gas at a specific temperature (its boiling point), forming bubbles throughout the liquid (e.g., water boiling into steam).
- Condensation: The process by which a gas changes into a liquid upon cooling (e.g., water vapor forming clouds).
- Sublimation: The direct change of a solid into a gas, or a gas into a solid, without passing through the liquid state (e.g., dry ice (solid carbon dioxide) turning directly into gaseous carbon dioxide).
Latent Heat & Effect of Temperature and Pressure
- Latent Heat: The “hidden” heat energy absorbed or released during a change of state at a constant temperature.
- Latent Heat of Fusion: The energy required to change 1 kg of a solid into a liquid at its melting point.
- Latent Heat of Vaporization: The energy required to change 1 kg of a liquid into a gas at its boiling point.
- Effect of Temperature and Pressure:
- Temperature: Increasing temperature generally causes matter to move towards states with higher particle energy (solid right arrow liquid right arrow gas). Decreasing temperature causes the opposite.
- Pressure: Increasing pressure generally favors states with closer particle packing (gas right arrow liquid right arrow solid). Decreasing pressure favors states with more spread-out particles.
Evaporation and Factors Affecting It
Evaporation is a surface phenomenon where liquid changes into vapor at any temperature below its boiling point. Unlike boiling, evaporation occurs only from the surface of the liquid.
Surface Area, Wind Speed, Temperature, Humidity
Several factors influence the rate of evaporation:
- Surface Area: A larger surface area exposed to the air allows more particles to escape, increasing the rate of evaporation. (e.g., clothes dry faster when spread out).
- Wind Speed: Increased wind speed carries away water vapor, preventing it from returning to the liquid state and thus increasing evaporation. (e.g., clothes dry faster on a windy day).
- Temperature: Higher temperatures provide more kinetic energy to liquid particles, enabling more of them to escape into the vapor phase, increasing evaporation. (e.g., water evaporates faster in the sun).
- Humidity: Humidity refers to the amount of water vapor present in the air. High humidity means the air already contains a significant amount of water vapor, reducing the rate at which more water can evaporate into it. (e.g., clothes dry slower on a humid day).
Practical Examples (Sweating, Cooling of Water in Earthen Pot)
- Sweating: When we sweat, the water on our skin evaporates. This process absorbs latent heat from our body, leading to a cooling sensation.
- Cooling of Water in an Earthen Pot: Earthen pots are porous, allowing some water to seep to the surface. This water then evaporates, taking latent heat from the remaining water inside the pot, thus cooling it.
| Property / State | Solid | Liquid | Gas |
| Shape | Definite | No definite (takes container’s shape) | No definite (fills container) |
| Volume | Definite | Definite | No definite |
| Compressibility | Negligible | Very low | High |
| Interparticle Space | Very little | More than solids, less than gases | Very large |
| Interparticle Force | Strong | Moderate | Weak |
| Rigidity/Fluidity | Rigid | Fluid | Fluid |
| Diffusion | Extremely slow (negligible) | Slower than gases, faster than solids | Very fast |
NCERT Solutions Class 9 Science Chapter 1 Important NCERT Textual Questions
1. Convert the following temperatures to the Celsius scale.
a) 293 K b) 470 K
A. Temperature on kelvin Scale = Temperature on Celsius scale + 273
a) 293 K = Temperature on Celsius scale + 273.
Temperature on Celsius scale = 293 – 273 = 20ºC
b) 470 K = Temperature on Celsius scale + 273.
Temperature on Celsius scale = 470 – 273 =197ºC
2. Convert the following temperature to the kelvin scale.
a) 25ºC b) 373ºC
A. a) Temperature on kelvin scale = Temperature on Celsius scale + 273 = 25 + 273 = 298 K
b) Temperature on kelvin scale = 373 + 273 = 646 K
3. Give reason for the following observations.
a. Naphthalene balls disappear with time without leaving any solid.
A. Naphthalene balls can sublime. Therefore, it changes into vapors completely, which disappear into the air and no solid is left. It is also called as sublimation.
b. Can we get the smell of perfume sitting several meters away.
A. Yes! it is because perfumes contain volatile solvent, which carries pleasant smelling vapors. They diffuse quite fast and can reach to people sitting several meters away.
4. Arrange the following substances in increasing order of forces of attraction between the particles of water, sugar, Oxygen.
A. The increasing order of inter molecular forces of attraction: Gas < Liquid < Solid
as well as, Oxygen < water < Sugar.
5. What is the physical state of water at.
a) 25ºC b) 0ºC c) 100ºC
A. a) Physical state of water at 25ºC is Liquid
b) Physical state of water at 0º C is liquid (or) solid
c) Physical state of water at 100ºC is liquid (or) gas
Note: An equilibrium will be existing between liquid and solid (or) liquid and gas
6. Give two reasons to justify:
a. Water at room temperature is a liquid
A. Water at room temperature is a liquid because
i) Water has a definite volume and has no definite shape. It acquires the shape of the containers.
ii) It has one free surface
iii) It can be flow
b. An iron almirah is a solid at room temperature.
A. i) Iron almirah is rigid and cannot be compressed
ii) It has definite shape and volume
7. Why is ice at 273 K more effective in cooling than water at the same temperature.
A. Cooling takes place, when heat is removed from a system.
In the case of ice at 273K, it will take latent heat from the medium to convert itself into water at 273 K, and then into water at higher temperature. Ice at 273 K, there will be change in physical state, where as in case of water at 273 K, there will be no change in state. So, particles in water at 273 K, have more energy compared to particles in ice at the same temperature.
8. What produces more severe burns, boiling water (or) steam?
A. Steam will produce more severe burns at 100ºC, than boiling water at the same temperature (100ºC)
9. Name A, B, C, D, E and F in the following diagram showing change in its state.
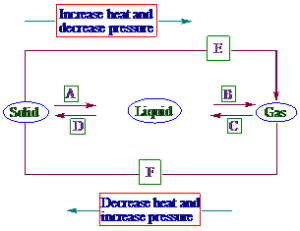
A = Fusion (or) Melting D = Solidification (or) Freezing
B = Vaporization (or) boiling E = Sublimation
C = Condensation (or) liquification F = Deposition
Class 9 Science Chapter 1 NCERT Textual Exercises
1. Which of the following are matter? Chair, air, live, smell, hate, almonds, thought, cold, cold drink, smell of perfume.
A. Chair, air, almonds, cold drink.
2. Give reasons for the following observation. The smell of hot sizzling food reaches you several meters away, but to get the smell from cold food you have to go close.
A. The rate of diffusion is faster at higher temperature than at lower temperature. The rate of diffusion of hot sizzling food is more and therefore, reaches us even several meters away. On the other hand, rate of diffusion of cold food is less and therefore, we have to go quite close to it in order to get its smell.
3. A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
A. The diver is able to cut through water in the swimming pool because matter is made up of particles which have vacant spaces. Between molecules of water, the inter molecular forces are not very strong. The diver can easily cut through water by applying force to displace water and occupy its place.
4. What are the characteristics of the particles of matter?
A. i) Particles of matter have space between them
ii) Particles of matter are continuously moving.
iii) Particles of matter attract each other.
5. The mass per unit volume of a substance is called density. [density = mass / volume]
Arrange the following in order of increasing density of air, exhaust from chimneys, honey, water, chalk, cotton and iron.
A. The order of increasing density is, air < exhaust from chimney < cotton < water < honey < chalk < iron
6. Tabulate the differences in the characteristics of states of matter:
A.
| S.No | Characteristics | Solid | Liquid | Gas |
| 1. | Mass | Definite | Definite | Definite |
| 2. | Shape | Definite | Not fixed. Acquires
the shape of container |
Not fixed. Acquires the shape of container |
| 3. | Volume | Not possible | Definite | Indefinite |
| 4. | Compressibility | Not possible | Slightly compressible | Highly compressible |
| 5. | Fluidity | Not possible | Can flow | Can flow |
| 6. | Diffusion | Slow | Fast | Very fast |
| 7. | Intermolecular force | Strongest | Slightly weaker than in solids | Negligible
(very very less) |
| 8. | Inter molecular spaces | Very less | large | very large |
7. Give reasons:
a. A gas fills completely the vessel in which it is kept:
A. Due to the force of attraction between particles of gas is very very less (negligible) and particles are free to move in all directions.
b. A gas exerts pressure on the walls of the container.
A. Gas molecules are free to move randomly in all directions. During their motion, they collide with one another and also with the walls of the container. The constant bombardment of the molecules on the walls of the container exerts a steady force. The force acting per unit area on the walls of the container is called pressure. Thus, gases exert pressure.
c. A wooden table should be called a solid.
A. A wooden table is called as solid because particles of wood are in fixed position and it has definite shape and volume.
d. We can easily move our hand in air but to do the same through a solid block of wood, we need a karate expert.
A. In air there is a lot of empty space between the molecules and the forces between the particles are very less. Hence, we can move our hand in air. Through a solid block of wood, only a Karate expert can do this because there are strong forces of attraction between molecules in a solid block of wood and there is no empty space between them.
8. Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find why?
A. Liquids generally have lower density than solids. But Ice has lower density than water because, it has an open cage like structure. In this structure, a lot of vacant spaces are left when water molecules are linked in ice. Because of lower density of ice, it floats on water.
9. Convert the following temperature to Celsius scale:
a) 300 K b) 573 K
A. a) Temperature on kelvin scale = temperature on Celsius scale + 273.
300 = Temperature on Celsius scale + 273.
Temperature on Celsius scale = 300 – 273 = 27ºC
b) Temperature on kelvin scale = temperature on Celsius scale + 273.
300 = Temperature on Celsius scale + 273.
Temperature on Celsius scale = 573 – 273 = 300ºC
10. What is the physical state of water at:
a) 250ºC b) 100ºC
A. a) At 250ºC, physical state of water is steam (or) gas
b) At 100ºC, Physical state of water is liquid (or) gas
Also Check: Most Easy and Scoring Chapters of CBSE Class 9 Science
11. Suggest a method to liquify atmospheric gases.
A. The atmospheric gas can be liquefied by cooling under pressure.
12. Why does a desert cooler cool better on a hot dry day?
A. It is due to evaporation process. A desert cooler cool better on a hot dry day, because the higher temperature on a hot day increases the rate of evaporation of water and the dryness of air also increases the rate of evaporation of water. That’s why, a desert cooler cools better on a hot dry day.

13. How does the water kept in an earthen pot (matka) become cool during Summer?
A. During summer water is kept in earthen wares to keep it cool because earthen wares (matka) has got a number of fine pores all around. water comes out through these pores and changes into vapor by taking heat from the inner water and atmosphere. Hence the water inside the Matka gets cooled.

14. Why are we able to sip hot tea or milk faster from a saucer rather than a cup?
A. Saucer has more surface area than cup, therefore rate of evaporation is more in case of saucer than the cup. Hence, more cooling will occur in the saucer. Hence, we are able to sip hot tea or milk faster from a saucer rather than a cup.

15. What type of clothes should we wear in summer and why?
A. In summer we perspire more. Therefore, to keep our body cool, we must wear cotton clothes. Since cotton clothes are good absorbers of water, they absorb the sweat quickly and expose it to the atmosphere for easy evaporation. Since evaporation produces cooling, cotton clothes help us in keeping our body cool.
Class 9 Science Chapter 1 Topic Notes
Chapter 1 – Matter in Our Surroundings
Unit 1: Matter – Its Nature and Behaviour include Chapter 1 – Matter in Our Surroundings. According to prior years’ question papers and past trends, this unit is worth 23 points out of a possible 100. As a result, it’s critical to spend time studying this chapter thoroughly.
The topics and subtopics from NCERT Solutions Class 9 Science Chapter 1 Matter in Our Surroundings are given below:
Physical nature of matter
The matter is Made Up of Particles
How Small Are These Particles of Matter?
Characteristic of particles of matter
Particles of Matter Have Space between Them
Particles of Matter Are Continuously Moving
Particles of Matter Attract Each Other
States of matter
The Solid State
The Liquid State
The Gaseous State
Can matter change its state
Effect of Change of Temperature
Effect of Change o Pressure
Evaporation
Factors Affecting Evaporation
How Does Evaporation Cause Cooling?
NCERT Solutions for Class 9 Science Chemistry Chapter 1 can be used as a quick reference to help students understand complex concepts.
From tiny sand particles on Earth to the enigmatic black holes at the centre of many galaxies, matter is one of the fundamental ingredients that make up everything in the universe. Matter plays a part in everything we see around us, interacting to create new materials that are both familiar and alien.
Discover the molecular components of matter and how it operates. Learn about the origins of the term matter and its relevance in various branches of research. To help you with your studies, look into more important NCERT Solutions for Class 9 Science.
NCERT Solutions for Class 9 Chapter 1: Matter in Our Surroundings includes exercises with question counts.
- Page 3 of Exercises 1.1 & 1.2 – Solutions to 4 Questions
- Page 6 of Exercise 1.3 – Solutions to 4 Questions
- Page 9 of Exercise 1.4 – Solutions to 4 Questions
- Page 10 of Exercise 1.5 – Solutions to 5 Questions
- Page 12 of Chapter Exercise – Solutions to 9 Questions
Key Features of NCERT Solutions for Class 9 Science Chapter 1
- The content was developed in great detail, with all jargon explained.
- Solutions have been crafted by skilled teachers and industry experts in an easy-to-understand manner.
- Questions from the most recent mandated curriculum are included.
- Exam questions from the previous year’s exam have been thoroughly examined.
- Additional learning resources, such as for example papers and previous year’s question papers, are available.
NCERT Solutions for Class 9 Science Chapter 1- FAQ’s
Explain the various state of matter properties discussed in Chapter 1 of NCERT Solutions for Class 9 Science.
Six criteria determine the distinct features of a state of matter: Shape Quantity Fluidity vs. Rigidity Intermolecular force Intermolecular space Compressibility The NCERT Solutions for Class 9 Science Chapter 1 curated by the specialists at INFINITY LEARN briefly explains these ideas. The solutions are written in simple language to make studying easy for the pupils.
In each exercise of NCERT Solutions for Class 9 Science Chapter 1, how many questions are there?
Each exercise in NCERT Solutions for Class 9 Science Chapter 1 has the following number of questions: 4 Questions for Exercises 1.1 and 1.2 4 Questions for Exercise 1.3 4 Questions for Exercise 1.4 5 Questions (Exercise 1.5) 9 Questions for Chapter Exercise
Is NCERT Solutions of Chapter 1 for Class 9 Science adequate for test preparation?
The experts at INFINITY LEARN created the NCERT Solutions for Class 9 Science Chapter 1 to enable students to ace the exam without anxiety. To boost student confidence, the key principles are given in the most systematic way possible. The NCERT Solutions address every last detail in order to aid students in their exam preparation. The solutions are available in both online and offline formats, allowing students to use them according to their needs.
What is the main topic covered in Class 9 Chapter 1 of the matter?
In Class 9 Chapter 1, the main topic is the introduction to matter, its definition, and the different states it can exist in.








