Table of Contents
Introduction to Anisole
Anisole is a fascinating compound that holds significant importance in the realm of organic chemistry. In this anisole article, we will delve into the captivating aspects of Anisole’s structure, synthesis, properties, and applications.
Anisole finds applications in diverse fields. As a solvent, it plays a significant role in the synthesis of pharmaceuticals, fragrances, and dyes. Its ability to dissolve both polar and nonpolar substances makes it valuable in different reaction conditions.
Anisole also contributes to the production of specific chemicals and materials, showcasing its importance in industrial processes. By understanding these aspects, we can unravel the complexities of this compound and appreciate its diverse range of applications.
Anisole Structure
Anisole, also known as methoxybenzene, is an organic compound that exhibits a unique and intriguing molecular structure. It consists of a benzene ring with a methoxy (-OCH3) group attached to it. This structural arrangement distinguishes anisole from other compounds, imparting distinct characteristics and behavior.
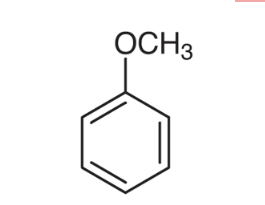
The Anisole formula is C7H8O, representing its seven carbon atoms, eight hydrogen atoms, and one oxygen atom. According to the IUPAC nomenclature, anisole iupac name is methoxybenzene.
Synthesis of Anisole: From Phenol to Anisole
One of the common methods of anisole synthesis is by converting a compound with a hydroxyl (-OH) group attached to a benzene ring, phenol to anisole.
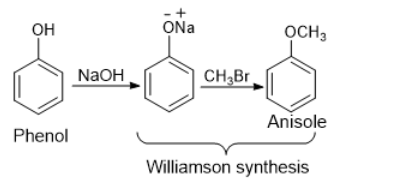
This two-step process converts phenol to anisole.
Step 1: Phenoxide ion formation
Phenoxde ion is formed when phenol is reacted with sodium hydroxide:
Step 2: Anisole Formation
The generated Phenoxide ion is now reacted with Methyl bromide (-CH3Br) to form Anisole.
As a result, when Phenol is treated with Sodium hydroxide, followed by a reaction with Methyl bromide, it produces Anisole.
Chemical reactions of Anisole
Anisole, also known as methoxybenzene, exhibits intriguing chemical reactivity.
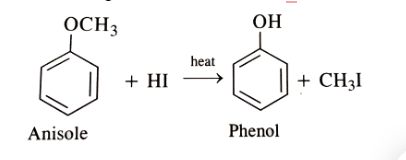
- Conversion of anisole to phenol, where the methoxy group (-OCH3) is replaced by a hydroxyl group (-OH). This transformation, known as demethylation, can be achieved through the following reaction of anisole + HI:
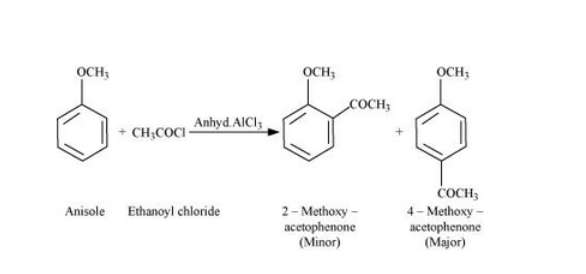
- Friedel Crafts acylation of Anisole, where an acyl group (R-C=O) is introduced onto the aromatic ring. This reaction requires an acylating agent, such as an acid chloride or acid anhydride, and a Lewis acid catalyst.
Properties of Anisole
- Anisole exhibits specific physical and chemical properties.
- Physically, it is a colorless liquid with a pleasant odor.
- It has a relatively low melting point of around -37 degrees Celsius and a boiling point of approximately 154 degrees Celsius.
- Anisole’s chemical properties allow it to undergo reactions such as electrophilic aromatic substitution and nucleophilic substitution, making it versatile in various chemical transformations.
Applications of Anisole
Anisole finds numerous applications in organic synthesis and industry.
It is commonly used as a solvent in the production of pharmaceuticals, fragrances, and dyes.
Its ability to dissolve both polar and nonpolar substances makes it valuable in different reaction conditions.
Anisole also plays a significant role in the synthesis of various chemical compounds, contributing to the development of new materials and substances.
Conclusion
Anisole, with its distinct structure, synthesis methods, properties, and applications, is a compound of great importance in organic chemistry. Understanding its structure and properties allows scientists to utilize its unique characteristics in various chemical processes.
Its applications in organic synthesis and industry showcase its versatility and relevance in numerous sectors. By exploring the world of Anisole, we gain valuable insights into the complex nature of organic compounds and their practical significance in the field of chemistry.
Frequently Asked Questions (FAQs) on Anisole
What is Anisole?
Anisole is an organic compound with the chemical formula C7H8O. It consists of a benzene ring attached to a methoxy (-OCH3) group. Anisole is commonly known as methoxybenzene and is used in various industrial applications.
How does Anisole react with Methyl Chloride?
Anisole can undergo various reactions with methyl chloride, depending on the reaction conditions. One common reaction is the nucleophilic aromatic substitution (S N Ar) reaction, where the methoxy group of Anisole is substituted by a methyl group from methyl chloride, resulting in the formation of methyl anisole.
How to convert Phenol to Anisole?
Phenol can be converted to Anisole through a process called acylation. One commonly used method is the Friedel-Crafts acylation reaction. In this reaction, Phenol reacts with an acid chloride or acid anhydride in the presence of a Lewis acid catalyst, such as aluminum chloride (AlCl3), to form Anisole.
How is Anisole obtained from Phenol?
Anisole can be obtained from Phenol through the process of methylation. Methylation involves the introduction of a methyl group (-CH3) onto the aromatic ring of Phenol. This can be achieved by reacting Phenol with a methylating agent, such as dimethyl sulfate or methyl iodide, under appropriate reaction conditions.
How do you convert Phenol to Anisole?
To convert Phenol to Anisole, one commonly employed method is the reaction with dimethyl sulfate. Phenol is mixed with dimethyl sulfate and heated under reflux. The reaction proceeds through an electrophilic aromatic substitution mechanism, resulting in the substitution of the hydroxyl group in Phenol with a methoxy group, thus forming Anisole.








